Clinical Management of Herpes Zoster in Patients With Rheumatoid Arthritis or Psoriatic Arthritis Receiving Tofacitinib Treatment
- PMID: 34870800
- PMCID: PMC8814083
- DOI: 10.1007/s40744-021-00390-0
Clinical Management of Herpes Zoster in Patients With Rheumatoid Arthritis or Psoriatic Arthritis Receiving Tofacitinib Treatment
Abstract
Introduction: Risk of herpes zoster (HZ) is increased with Janus kinase inhibitor use. We evaluated clinical study data relating to HZ management in patients with rheumatoid arthritis (RA) or psoriatic arthritis (PsA) receiving tofacitinib.
Methods: This post hoc analysis included data from 21 RA and 3 PsA clinical studies; data were pooled for tofacitinib doses. Outcomes of HZ events (serious and non-serious) and tofacitinib treatment changes were evaluated in response to first and second HZ events. Median time to resolution was stratified by dermatomal involvement, history of HZ prior to tofacitinib, changes to tofacitinib treatment, anti-viral and corticosteroid use, and tofacitinib dose.
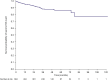
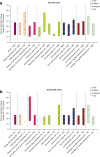
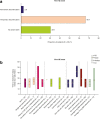
Results: Seven hundred eighty-three (11.1%, N = 7061) patients with RA experienced ≥ 1 HZ event, 63 (8.0%) of whom had ≥ 2 HZ events. In patients with PsA, 36 (4.6%, N = 783) experienced ≥ 1 HZ event, 1 (2.8%) of whom had ≥ 2 HZ events. For most HZ events, tofacitinib treatment was unchanged or temporarily discontinued. The majority of patients received anti-viral treatment, most within 3 days of onset. Post-herpetic neuralgia developed in 6.9% and 3.2% of patients with RA with first and second events, respectively, and in 2.8% of patients with PsA with a first event. Most first and second events resolved (RA: 97.6% and 96.8%, respectively; PsA: 94.4% and 100%, respectively). Median time to resolution was 22.0 days for first and 15.0 days for second events for RA and 20.5 days for first and 11.0 days for second events (n = 1) for PsA. Time to resolution of first events for RA and PsA was generally numerically shorter for patients with single dermatomal HZ, history of HZ, or anti-viral use versus those without.
Conclusion: Among patients receiving tofacitinib, recurrent events were more common in patients with RA versus PsA; HZ duration was shorter for repeat events.
Trial registration: NCT01262118, NCT01484561, NCT00147498, NCT00413660, NCT00550446, NCT00603512, NCT00687193, NCT01164579, NCT00976599, NCT01059864, NCT01359150, NCT02147587, NCT00960440, NCT00847613, NCT00814307, NCT00856544, NCT00853385, NCT01039688, NCT02187055, NCT00413699, NCT00661661, NCT01877668, NCT01882439, NCT01976364.
Keywords: Anti-viral; Clinical management; Herpes zoster; Infections; Psoriatic arthritis; Rheumatoid arthritis; Tofacitinib.
Plain language summary
Patients with rheumatoid arthritis (RA) or psoriatic arthritis (PsA) have weakened immune responses and are more likely to get herpes zoster (HZ; also known as shingles) infections compared with the general population. Patients who receive treatments for RA or PsA that have an effect on their immune system are more likely to get HZ. Here, we assessed how common HZ was in patients with RA or PsA who were given tofacitinib during clinical trials, the management of these infections, and how this affected the course of the infection. Approximately 1 in 10 patients with RA and 1 in 20 patients with PsA had HZ. Of those patients who had HZ, 1 in 12 with RA and 1 in 36 with PsA were infected again at a later point. A small number of patients also had long-lasting pain after HZ infection. When patients had a HZ infection, most either continued treatment with tofacitinib or paused treatment for a period of time. Pausing or continuing treatment did not appear to affect how long the infection lasted or whether patients had another infection. Most patients received treatment for HZ infection, and patients who were treated had shorter infections. In most patients, infections cleared up and were more likely to clear up more quickly when patients had HZ previously.
© 2021. The Author(s).
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

