Generation of Pericytic-Vascular Progenitors from Tankyrase/PARP-Inhibitor-Regulated Naïve (TIRN) Human Pluripotent Stem Cells
- PMID: 34870835
- PMCID: PMC9529319
- DOI: 10.1007/978-1-0716-1908-7_10
Generation of Pericytic-Vascular Progenitors from Tankyrase/PARP-Inhibitor-Regulated Naïve (TIRN) Human Pluripotent Stem Cells
Abstract
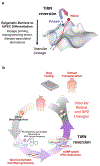
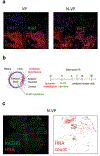
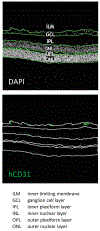
Tankyrase/PARP inhibitor-regulated naïve human pluripotent stem cells (TIRN-hPSC) represent a new class of human stem cells for regenerative medicine that can differentiate into multi-lineage progenitors with improved in vivo functionality. Chemical reversion of conventional, primed hPSC to a TIRN-hPSC state alleviates dysfunctional epigenetic donor cell memory, lineage-primed gene expression, and potentially disease-associated aberrations in their differentiated progeny. Here, we provide methods for the reversion of normal or diseased patient-specific primed hPSC to TIRN-hPSC and describe their subsequent differentiation into embryonic-like pericytic-endothelial "naïve" vascular progenitors (N-VP). N-VP possess improved vascular functionality, high epigenetic plasticity, maintain greater genomic stability, and are more efficient in migrating to and re-vascularizing ischemic tissues than those generated from primed isogenic hPSC. We also describe detailed methods for the ocular transplantation and quantitation of vascular engraftment of N-VP into the ischemia-damaged neural retina of a humanized mouse model of ischemic retinopathy. The application of TIRN-hPSC-derived N-VP will advance vascular cell therapies of ischemic retinopathy, myocardial infarction, and cerebral vascular stroke.
Keywords: Differentiation; Human pluripotent stem cell; Ischemic retinopathy; Naïve pluripotency; PARP; Pericyte; Tankyrase inhibition; Vascular progenitors; Vascular regeneration.
© 2022. The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

