Catalysis-Enabled Concise and Stereoselective Total Synthesis of the Tricyclic Prostaglandin D2 Metabolite Methyl Ester
- PMID: 34870881
- PMCID: PMC8766936
- DOI: 10.1002/anie.202115633
Catalysis-Enabled Concise and Stereoselective Total Synthesis of the Tricyclic Prostaglandin D2 Metabolite Methyl Ester
Abstract
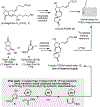
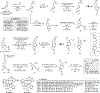
A concise and stereoselective total synthesis of the clinically relevant tricyclic prostaglandin D2 metabolite (tricyclic-PGDM) methyl ester in racemic form was accomplished in eight steps from a readily available known cyclopentene-diol derivative. The synthesis features a nickel-catalyzed Ueno-Stork-type dicarbofunctionalization to generate two consecutive stereocenters, a palladium-catalyzed carbonylative spirolactonization to build the core oxaspirolactone, and a Z-selective cross-metathesis to introduce the (Z)-3-butenoate side chain, a group challenging to introduce through traditional Wittig protocols and troublesome for the two previous total syntheses. A general Z-selective cross-metathesis protocol to construct (Z)-β,γ-unsaturated esters was also developed that has broad functional group tolerance and high stereoselectivity. Additionally, our synthesis already accumulated 75 mg of valuable material for an 18 O-tricyclic-PGDM-based assay used in clinical settings for inflammation.
Keywords: Carbonylation; Nickel catalysis; Olefin metathesis; Prostaglandins; Total synthesis.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
Conflicts of interest
The authors declare no conflicts of interest.
Figures

References
-
- FitzGerald GA, Nat. Rev. Drug Discov, 2003, 2, 879–890. - PubMed
-
-
For selected examples: Urade Y, Hayaishi O, Sleep Med. Rev, 2011, 15, 411–418;Lee ML, Matsunaga H, Sugiura Y, Hayasaka T, Yamamoto I, Ishimoto T, Imoto D, Suematsu M, Iijima N, Kimura K, Diano S, Toda C, Nat. Commun, 2021, 12, 2330;Palla AR, Ravichandran M, Wang YX, Alexandrova L, Yang AV, Kraft P, Holbrook CA, Schürch CM, Ho ATV, Blau HM, Science, 2021, 371, eabc8059.
-
-
- Tsikas D, Chromatogr J B Biomed. Sci. Appl, 1998, 717, 201–245. - PubMed
-
- Liston TE, Roberts LJ, J. Biol. Chem, 1985, 260, 13172–13180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

