Acquired Clinical Immunity to Malaria in Nonhuman Primates Coinfected with Schistosoma and Plasmodium Parasites
- PMID: 34871040
- PMCID: PMC8853685
- DOI: 10.1128/IAI.00464-21
Acquired Clinical Immunity to Malaria in Nonhuman Primates Coinfected with Schistosoma and Plasmodium Parasites
Abstract
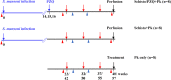
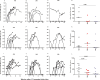
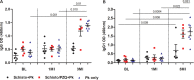
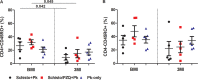
Naturally acquired immunity to malaria develops over several years and can be compromised by concomitant infections. This study explored the influence of chronic schistosomiasis on clinical outcome and immunity to repeated malaria infection. Two groups of baboons (n = 8 each), were infected with Schistosoma mansoni cercariae to establish chronic infections. One of the two groups was treated with praziquantel (PZQ) to eliminate schistosome infection. The two groups plus a new malaria control group (n = 8) were inoculated three times with Plasmodium knowlesi parasites at 1-month intervals. Clinical data and IgG, IgG1, memory T-cell, and monocyte levels were recorded. After three P. knowlesi infections, we observed (i) reduced clinical symptoms in all groups with each subsequent infection, (ii) increased IgG and IgG1 levels in the malaria control (Pk-only) group, (iii) increased IgG, IgG1, CD14+, and CD14- CD16+ levels in the Schistosoma-treated (Schisto/PZQ+Pk) group, and (iv) significantly lower IgG and IgG1 levels compared to those of the Pk-only group, reduced CD4+ CD45RO+ levels, and increased levels of CD14- CD16+ cells in the coinfected (Schisto+Pk) group. Chronic S. mansoni infection does not compromise establishment of clinical immunity after multiple malaria infections, with nonclassical monocytes seeming to play a role. Failure to develop robust antibody and memory T cells may have a long-term impact on acquired immunity to malaria infection.
Keywords: acquired immunity; coinfection; malaria; schistosomiasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization. 2020. World malaria report 2019. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

