Combination of Pharmacokinetic and Pathogen Susceptibility Information To Optimize Meropenem Treatment of Gram-Negative Infections in Critically Ill Patients
- PMID: 34871092
- PMCID: PMC8846453
- DOI: 10.1128/AAC.01831-21
Combination of Pharmacokinetic and Pathogen Susceptibility Information To Optimize Meropenem Treatment of Gram-Negative Infections in Critically Ill Patients
Abstract
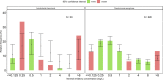
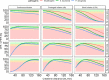
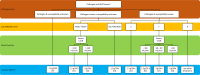
Meropenem is one of the most frequently used antibiotics to treat life-threatening infections in critically ill patients. This study aimed to develop a meropenem dosing algorithm for the treatment of Gram-negative infections based on intensive care unit (ICU)-specific resistance data. Antimicrobial susceptibility testing of Gram-negative bacteria obtained from critically ill patients was carried out from 2016 to 2020 at a tertiary care hospital. Based on the observed MIC distribution, stochastic simulations (n = 1,000) of an evaluated pharmacokinetic meropenem model, and a defined pharmacokinetic/pharmacodynamic target (100%T>4×MIC while minimum concentrations were <44.5 mg/L), dosing recommendations for patients with varying renal function were derived. Pathogen-specific MIC distributions were used to calculate the cumulative fraction of response (CFR), and the overall MIC distribution was used to calculate the local pathogen-independent mean fraction of response (LPIFR) for the investigated dosing regimens. A CFR/LPIFR of >90% was considered adequate. The observed MIC distribution significantly differed from the EUCAST database. Based on the 6,520 MIC values included, a three-level dosing algorithm was developed. If the pathogen causing the infection is unknown (level 1), known (level 2), known to be neither Pseudomonas aeruginosa nor Acinetobacter baumannii, or classified as susceptible (level 3), a continuous infusion of 1.5 g daily reached sufficient target attainment independent of renal function. In all other cases, dosing needs to be adjusted based on renal function. ICU-specific susceptibility data should be assessed regularly and integrated into dosing decisions. The presented workflow may serve as a blueprint for other antimicrobial settings.
Keywords: Gram negative; antimicrobial susceptibility testing; critically ill; dosing algorithm; meropenem.
Conflict of interest statement
The authors declare a conflict of interest. CK reports grants from an industry consortium (AbbVie Deutschland GmbH & Co. KG, AstraZeneca, Boehringer Ingelheim Pharma GmbH & Co. KG, Grünenthal GmbH, F. Hoffmann-La Roche Ltd, Merck KGaA and SANOFI) for the PharMetrX program, grants from the Innovative Medicines Initiative-Joint Undertaking ("DDMoRe"), Diurnal Ltd., the Federal Ministry of Education and Research within the Joint Programming Initiative on Antimicrobial Resistance Initiative (JPIAMR) and the European Commission within in the Horizon 2020 framework programme ("FAIR"), all outside the submitted work.
C.K. reports grants from an industry consortium (AbbVie Deutschland GmbH & Co. KG; AstraZeneca; Boehringer Ingelheim Pharma GmbH & Co. KG; Grünenthal GmbH; F. Hoffmann-La Roche, Ltd.; Merck KGaA; and SANOFI) for the PharMetrX program and grants from the Innovative Medicines Initiative-Joint Undertaking (DDMoRe), Diurnal, Ltd., the Federal Ministry of Education and Research within the Joint Programming Initiative on Antimicrobial Resistance Initiative (JPIAMR), and the European Commission within the Horizon 2020 framework program “FAIR,” all outside the present work.
Figures
Similar articles
-
Development of a dosing algorithm for meropenem in critically ill patients based on a population pharmacokinetic/pharmacodynamic analysis.Int J Antimicrob Agents. 2019 Sep;54(3):309-317. doi: 10.1016/j.ijantimicag.2019.06.016. Epub 2019 Jun 20. Int J Antimicrob Agents. 2019. PMID: 31229669
-
Time Above All Else: Pharmacodynamic Analysis of β-Lactams in Critically Ill Patients.J Clin Pharmacol. 2022 Apr;62(4):479-485. doi: 10.1002/jcph.1977. Epub 2021 Nov 12. J Clin Pharmacol. 2022. PMID: 34614542
-
Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: a prospective observational study.Crit Care. 2017 Oct 21;21(1):263. doi: 10.1186/s13054-017-1829-4. Crit Care. 2017. PMID: 29058601 Free PMC article. Clinical Trial.
-
Meropenem for the Pharmacological Treatment of Severe Infections in Critically Ill Pediatric Patients: Breakthrough Standard Treatment Strategies Based on PK/PD.Curr Drug Metab. 2023;24(1):5-15. doi: 10.2174/1389200224666230325121729. Curr Drug Metab. 2023. PMID: 36974414 Review.
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
Cited by
-
Population Pharmacokinetics of Vancomycin in Intensive Care Patients with the Time-Varying Status of Temporary Mechanical Circulatory Support or Continuous Renal Replacement Therapy.Infect Dis Ther. 2024 Dec;13(12):2617-2635. doi: 10.1007/s40121-024-01071-5. Epub 2024 Nov 14. Infect Dis Ther. 2024. PMID: 39541003 Free PMC article.
-
Evaluation of a Meropenem and Piperacillin Monitoring Program in Intensive Care Unit Patients Calls for the Regular Assessment of Empirical Targets and Easy-to-Use Dosing Decision Tools.Antibiotics (Basel). 2022 Jun 2;11(6):758. doi: 10.3390/antibiotics11060758. Antibiotics (Basel). 2022. PMID: 35740164 Free PMC article.
-
Software- and TDM-Guided Dosing of Meropenem Promises High Rates of Target Attainment in Critically Ill Patients.Antibiotics (Basel). 2023 Jun 27;12(7):1112. doi: 10.3390/antibiotics12071112. Antibiotics (Basel). 2023. PMID: 37508207 Free PMC article.
-
Antimicrobial susceptibility at intensive care units in Sudan, antibiogram development.BMC Microbiol. 2025 May 14;25(1):290. doi: 10.1186/s12866-025-04021-4. BMC Microbiol. 2025. PMID: 40369426 Free PMC article.
-
What's new in therapeutic drug monitoring of antimicrobials?Intensive Care Med. 2023 Jul;49(7):857-859. doi: 10.1007/s00134-023-07060-5. Epub 2023 May 3. Intensive Care Med. 2023. PMID: 37133741 Free PMC article. No abstract available.
References
-
- Engel C, Brunkhorst FM, Bone H-G, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, Mayer K, Oppert M, Olthoff D, Quintel M, Ragaller M, Rossaint R, Stuber F, Weiler N, Welte T, Bogatsch H, Hartog C, Loeffler M, Reinhart K. 2007. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med 33:606–618. 10.1007/s00134-006-0517-7. - DOI - PubMed
-
- Vincent J-L, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, EPIC II Group of Investigators. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329. 10.1001/jama.2009.1754. - DOI - PubMed
-
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. 10.1097/01.CCM.0000217961.75225.E9. - DOI - PubMed
-
- Pfizer. 2019. Meropenem. Summary of product characteristics. https://www.pfizer.de/sites/default/files/FI-16053.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

