Randomized, double-blind, controlled trial of human anti-LIGHT monoclonal antibody in COVID-19 acute respiratory distress syndrome
- PMID: 34871182
- PMCID: PMC8803341
- DOI: 10.1172/JCI153173
Randomized, double-blind, controlled trial of human anti-LIGHT monoclonal antibody in COVID-19 acute respiratory distress syndrome
Abstract
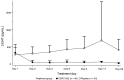
BACKGROUNDSevere coronavirus disease 2019 (COVID-19) is associated with a dysregulated immune response, which can result in cytokine-release syndrome and acute respiratory distress syndrome (ARDS). Patients with COVID-19-associated ARDS have elevated free serum levels of the cytokine lymphotoxin-like inducible protein that competes with glycoprotein D for herpesvirus entry on T cells (LIGHT; also known as TNFSF14). Such patients may benefit from LIGHT-neutralization therapy.METHODSThis randomized, double-blind, multicenter, proof-of-concept trial enrolled adults hospitalized with COVID-19-associated pneumonia and mild to moderate ARDS. Patients received standard of care plus a single dose of a human LIGHT-neutralizing antibody (CERC-002) or placebo. The primary endpoint was the proportion of patients receiving CERC-002 who remained alive and free of respiratory failure through day 28. Safety was assessed via adverse event monitoring.RESULTSFor most of the 83 enrolled patients, standard of care included systemic corticosteroids (88.0%) or remdesivir (57.8%). A higher proportion of patients remained alive and free of respiratory failure through day 28 after receiving CERC-002 (83.9%) versus placebo (64.5%; P = 0.044), including in patients 60 years of age or older (76.5% vs. 47.1%, respectively; P = 0.042). Mortality rates were 7.7% (CERC-002) and 14.3% (placebo) on day 28 and 10.8% and 22.5%, respectively, on day 60. Treatment-emergent adverse events were less frequent with CERC-002 than placebo.CONCLUSIONFor patients with COVID-19-associated ARDS, adding CERC-002 to standard-of-care treatment reduces LIGHT levels and might reduce the risk of respiratory failure and death.TRIAL REGISTRATIONClinicalTrials.gov NCT04412057.FUNDINGAvalo Therapeutics.
Keywords: Adaptive immunity; COVID-19; Clinical Trials; Cytokines; Respiration.
Conflict of interest statement
Figures


References
-
- Parr J. Pneumonia in China: lack of information raises concerns among Hong Kong health workers. BMJ. 2020;368:m56. - PubMed
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int Updated December 7, 2021. Accessed December 7, 2021.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

