Efficacy and safety of combination therapy with pramipexole and levodopa vs levodopa monotherapy in patients with Parkinson disease: A systematic review and meta-analysis
- PMID: 34871213
- PMCID: PMC8568447
- DOI: 10.1097/MD.0000000000027511
Efficacy and safety of combination therapy with pramipexole and levodopa vs levodopa monotherapy in patients with Parkinson disease: A systematic review and meta-analysis
Abstract
Background: Pramipexole (P) or levodopa (L) treatment has been suggested as a therapeutic method for Parkinson disease (PD) in many clinical studies. Nonetheless, the combined effects of 2 drugs for PD patients are not completely understood.The aim of this research was to evaluate the clinical efficacy and safety of P plus L (P+L) combination therapy in the treatment of PD compared to that of L monotherapy, in order to confer a reference for clinical practice.
Methods: Randomized controlled trials (RCTs) of P+L for PD published up to April, 2020 were retrieved. Standardized mean difference (SMD), odds ratio (OR), and 95% confidence interval (CI) were calculated and heterogeneity was measured with the I2 test. Sensitivity analysis was also carried out. The outcomes of interest were as follows: the efficacy, unified Parkinson disease rating scale (UPDRS) scores, Hamilton depression rating scale score or adverse events.
Results: Twenty-four RCTs with 2171 participants were included. Clinical efficacy of P+L combination therapy was significantly better than L monotherapy (9 trials; OR 4.29, 95% CI 2.78 to 6.64, P < .00001). Compared with L monotherapy, the pooled effects of P+L combination therapy on UPDRS score were (22 trials; SMD -1.31, 95% CI -1.57 to -1.04, P < .00001) for motor UPDRS score, (16 trials; SMD -1.26, 95% CI -1.49 to -1.03, P < .00001) for activities of daily living UPDRS score, (12 trials; SMD -1.02, 95% CI -1.27 to -0.77, P < .00001) for mental UPDRS score, (10 trials; SMD -1.54, 95% CI -1.93 to -1.15, P < .00001) for complication UPDRS score. The Hamilton depression rating scale score showed significant decrease in the P+L combination therapy compared to L monotherapy (12 trials; SMD -1.56, 95% CI -1.90 to -1.22, P < .00001). In contrast to L monotherapy, P+L combination therapy reduced the number of any adverse events obviously in PD patients (16 trials; OR 0.36, 95% CI 0.27 to 0.50, P < .00001).
Conclusions: P+L combination therapy is superior to L monotherapy for improvement of clinical symptoms in PD patients. Moreover, the safety profile of P+L combination therapy is better than that of L monotherapy. Further well-designed, multicenter RCTs needed to identify these findings.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures
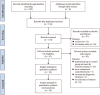

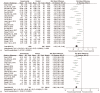



Similar articles
-
Comparison of pramipexole and levodopa/benserazide combination therapy versus levodopa/benserazide monotherapy in the treatment of Parkinson's disease: a systematic review and meta-analysis.Naunyn Schmiedebergs Arch Pharmacol. 2021 Sep;394(9):1893-1905. doi: 10.1007/s00210-021-02089-z. Epub 2021 May 7. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33959780
-
Comparison of selegiline and levodopa combination therapy versus levodopa monotherapy in the treatment of Parkinson's disease: a meta-analysis.Aging Clin Exp Res. 2020 May;32(5):769-779. doi: 10.1007/s40520-019-01232-4. Epub 2019 Jun 7. Aging Clin Exp Res. 2020. PMID: 31175606 Review.
-
Rasagiline combined with levodopa therapy versus levodopa monotherapy for patients with Parkinson's disease: a systematic review.Neurol Sci. 2020 Jan;41(1):101-109. doi: 10.1007/s10072-019-04050-8. Epub 2019 Aug 24. Neurol Sci. 2020. PMID: 31446579
-
Dilemma in Parkinson's Treatment; Levodopa Monotherapy May be the Best Choice.J Clin Neurosci. 2020 Sep;79:219-223. doi: 10.1016/j.jocn.2020.06.024. Epub 2020 Aug 6. J Clin Neurosci. 2020. PMID: 33070900
-
Adding a dopamine agonist to preexisting levodopa therapy vs. levodopa therapy alone in advanced Parkinson's disease: a meta analysis.Int J Clin Pract. 2009 Apr;63(4):613-23. doi: 10.1111/j.1742-1241.2009.02027.x. Epub 2009 Feb 16. Int J Clin Pract. 2009. PMID: 19222614
Cited by
-
Molecular pathways: the quest for effective MAO-B inhibitors in neurodegenerative therapy.Mol Biol Rep. 2025 Feb 17;52(1):240. doi: 10.1007/s11033-025-10349-x. Mol Biol Rep. 2025. PMID: 39961877 Review.
-
Real-World Prescription Patterns For Patients With Young-Onset Parkinson's Disease in China: A Trend Analysis From 2014 to 2019.Front Pharmacol. 2022 May 12;13:858139. doi: 10.3389/fphar.2022.858139. eCollection 2022. Front Pharmacol. 2022. PMID: 35645835 Free PMC article.
-
Impact of Physical Exercise on Levodopa Therapy Across Parkinson's Disease Stages.J Parkinsons Dis. 2024;14(5):1039-1049. doi: 10.3233/JPD-230384. J Parkinsons Dis. 2024. PMID: 38905055 Free PMC article.
-
A Systematic Review and Meta-Analysis of the Efficacy and Safety of Rasagiline or Pramipexole in the Treatment of Early Parkinson's Disease.Parkinsons Dis. 2024 Jan 16;2024:8448584. doi: 10.1155/2024/8448584. eCollection 2024. Parkinsons Dis. 2024. PMID: 38264500 Free PMC article. Review.
References
-
- Stutts LA, Speight KL, Yoo S, et al. . Positive psychological predictors of psychological health in individuals with Parkinson's disease. J Clin Psychol Med Settings 2020;27:182–9. - PubMed
-
- Jiang DQ, Wang HK, Wang Y, et al. . Rasagiline combined with levodopa therapy versus levodopa monotherapy for patients with Parkinson's disease: a systematic review. Neurol Sci 2020;41:101–9. - PubMed
-
- Gupta HV, Lyons KE, Wachter N, et al. . Long term response to levodopa in Parkinson's disease. J Parkinsons Dis 2019;9:525–9. - PubMed
-
- Delamarre A, Tison F, Li Q, et al. . Assessment of plasma creatine kinase as biomarker for levodopa-induced dyskinesia in Parkinson's disease. J Neural Transm (Vienna) 2019;126:789–93. - PubMed
-
- Jiang DQ, Jiang LL, Wang Y, et al. . The role of pramipexole in the treatment of patients with depression and Parkinson's disease: a meta-analysis of randomized controlled trials. Asian J Psychiatr 2021;61:102691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

