Simulations of dynamically cross-linked actin networks: Morphology, rheology, and hydrodynamic interactions
- PMID: 34871298
- PMCID: PMC8675935
- DOI: 10.1371/journal.pcbi.1009240
Simulations of dynamically cross-linked actin networks: Morphology, rheology, and hydrodynamic interactions
Abstract
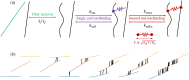
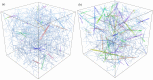
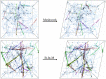
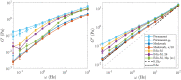
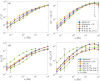
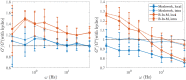
Cross-linked actin networks are the primary component of the cell cytoskeleton and have been the subject of numerous experimental and modeling studies. While these studies have demonstrated that the networks are viscoelastic materials, evolving from elastic solids on short timescales to viscous fluids on long ones, questions remain about the duration of each asymptotic regime, the role of the surrounding fluid, and the behavior of the networks on intermediate timescales. Here we perform detailed simulations of passively cross-linked non-Brownian actin networks to quantify the principal timescales involved in the elastoviscous behavior, study the role of nonlocal hydrodynamic interactions, and parameterize continuum models from discrete stochastic simulations. To do this, we extend our recent computational framework for semiflexible filament suspensions, which is based on nonlocal slender body theory, to actin networks with dynamic cross linkers and finite filament lifetime. We introduce a model where the cross linkers are elastic springs with sticky ends stochastically binding to and unbinding from the elastic filaments, which randomly turn over at a characteristic rate. We show that, depending on the parameters, the network evolves to a steady state morphology that is either an isotropic actin mesh or a mesh with embedded actin bundles. For different degrees of bundling, we numerically apply small-amplitude oscillatory shear deformation to extract three timescales from networks of hundreds of filaments and cross linkers. We analyze the dependence of these timescales, which range from the order of hundredths of a second to the actin turnover time of several seconds, on the dynamic nature of the links, solvent viscosity, and filament bending stiffness. We show that the network is mostly elastic on the short time scale, with the elasticity coming mainly from the cross links, and viscous on the long time scale, with the effective viscosity originating primarily from stretching and breaking of the cross links. We show that the influence of nonlocal hydrodynamic interactions depends on the network morphology: for homogeneous meshworks, nonlocal hydrodynamics gives only a small correction to the viscous behavior, but for bundled networks it both hinders the formation of bundles and significantly lowers the resistance to shear once bundles are formed. We use our results to construct three-timescale generalized Maxwell models of the networks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Interplay between Brownian motion and cross-linking controls bundling dynamics in actin networks.Biophys J. 2022 Apr 5;121(7):1230-1245. doi: 10.1016/j.bpj.2022.02.030. Epub 2022 Feb 20. Biophys J. 2022. PMID: 35196512 Free PMC article.
-
Morphological transitions of elastic filaments in shear flow.Proc Natl Acad Sci U S A. 2018 Sep 18;115(38):9438-9443. doi: 10.1073/pnas.1805399115. Epub 2018 Sep 4. Proc Natl Acad Sci U S A. 2018. PMID: 30181295 Free PMC article.
-
Hydrodynamic interactions significantly alter the dynamics of actin networks and result in a length scale dependent loss modulus.J Biomech. 2021 May 7;120:110352. doi: 10.1016/j.jbiomech.2021.110352. Epub 2021 Mar 2. J Biomech. 2021. PMID: 33756413
-
Determinants of fluidlike behavior and effective viscosity in cross-linked actin networks.Biophys J. 2014 Feb 4;106(3):526-34. doi: 10.1016/j.bpj.2013.12.031. Biophys J. 2014. PMID: 24507593 Free PMC article.
-
Theory of Semiflexible Filaments and Networks.Polymers (Basel). 2017 Feb 5;9(2):52. doi: 10.3390/polym9020052. Polymers (Basel). 2017. PMID: 30970730 Free PMC article. Review.
Cited by
-
Interplay between Brownian motion and cross-linking controls bundling dynamics in actin networks.Biophys J. 2022 Apr 5;121(7):1230-1245. doi: 10.1016/j.bpj.2022.02.030. Epub 2022 Feb 20. Biophys J. 2022. PMID: 35196512 Free PMC article.
References
-
- Mofrad MR. Rheology of the cytoskeleton. Annual Review of Fluid Mechanics. 2009;41:433–453. doi: 10.1146/annurev.fluid.010908.165236 - DOI
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. Garland Science; 2002.
-
- Hinner B, Tempel M, Sackmann E, Kroy K, Frey E. Entanglement, elasticity, and viscous relaxation of actin solutions. Physical Review Letters. 1998;81(12):2614. doi: 10.1103/PhysRevLett.81.2614 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

