Deeplasmid: deep learning accurately separates plasmids from bacterial chromosomes
- PMID: 34871418
- PMCID: PMC8860608
- DOI: 10.1093/nar/gkab1115
Deeplasmid: deep learning accurately separates plasmids from bacterial chromosomes
Abstract
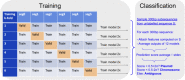
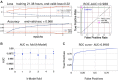
Plasmids are mobile genetic elements that play a key role in microbial ecology and evolution by mediating horizontal transfer of important genes, such as antimicrobial resistance genes. Many microbial genomes have been sequenced by short read sequencers and have resulted in a mix of contigs that derive from plasmids or chromosomes. New tools that accurately identify plasmids are needed to elucidate new plasmid-borne genes of high biological importance. We have developed Deeplasmid, a deep learning tool for distinguishing plasmids from bacterial chromosomes based on the DNA sequence and its encoded biological data. It requires as input only assembled sequences generated by any sequencing platform and assembly algorithm and its runtime scales linearly with the number of assembled sequences. Deeplasmid achieves an AUC-ROC of over 89%, and it was more accurate than five other plasmid classification methods. Finally, as a proof of concept, we used Deeplasmid to predict new plasmids in the fish pathogen Yersinia ruckeri ATCC 29473 that has no annotated plasmids. Deeplasmid predicted with high reliability that a long assembled contig is part of a plasmid. Using long read sequencing we indeed validated the existence of a 102 kb long plasmid, demonstrating Deeplasmid's ability to detect novel plasmids.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Martínez-Martínez L., Pascual A., Jacoby G.A.. Quinolone resistance from a transferable plasmid. Lancet. 1998; 351:797–799. - PubMed
-
- Klaenhammer T.R. Plasmid-directed mechanisms for bacteriophage defense in lactic streptococci. FEMS Microbiol. Rev. 1987; 3:313–325.
-
- Sing W.D., Klaenhammer T.R.. Characteristics of phage abortion conferred in lactococci by the conjugal plasmid pTR2030. Microbiology. 1990; 136:1807–1815.
-
- Silver S., Misra T.K.. Plasmid-mediated heavy metal resistances. Annu. Rev. Microbiol. 1988; 42:717–743. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

