VID22 counteracts G-quadruplex-induced genome instability
- PMID: 34871443
- PMCID: PMC8682794
- DOI: 10.1093/nar/gkab1156
VID22 counteracts G-quadruplex-induced genome instability
Abstract
Genome instability is a condition characterized by the accumulation of genetic alterations and is a hallmark of cancer cells. To uncover new genes and cellular pathways affecting endogenous DNA damage and genome integrity, we exploited a Synthetic Genetic Array (SGA)-based screen in yeast. Among the positive genes, we identified VID22, reported to be involved in DNA double-strand break repair. vid22Δ cells exhibit increased levels of endogenous DNA damage, chronic DNA damage response activation and accumulate DNA aberrations in sequences displaying high probabilities of forming G-quadruplexes (G4-DNA). If not resolved, these DNA secondary structures can block the progression of both DNA and RNA polymerases and correlate with chromosome fragile sites. Vid22 binds to and protects DNA at G4-containing regions both in vitro and in vivo. Loss of VID22 causes an increase in gross chromosomal rearrangement (GCR) events dependent on G-quadruplex forming sequences. Moreover, the absence of Vid22 causes defects in the correct maintenance of G4-DNA rich elements, such as telomeres and mtDNA, and hypersensitivity to the G4-stabilizing ligand TMPyP4. We thus propose that Vid22 is directly involved in genome integrity maintenance as a novel regulator of G4 metabolism.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

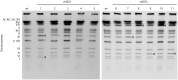
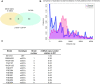
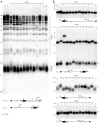
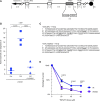
References
-
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993; 362:709–715. - PubMed
-
- Hoeijmakers J.H.J. DNA damage, aging, and cancer. N. Engl. J. Med. 2009; 361:1475–1485. - PubMed
-
- Bartek J., Lukas J.. DNA damage checkpoints: from initiation to recovery or adaptation. Curr. Opin. Cell Biol. 2007; 19:238–245. - PubMed
-
- Suijkerbuijk S.J.E., Kops G.J.P.L.. Preventing aneuploidy: the contribution of mitotic checkpoint proteins. Biochim. Biophys. Acta - Rev. Cancer. 2008; 1786:24–31. - PubMed
-
- Lazzaro F., Giannattasio M., Puddu F., Granata M., Pellicioli A., Plevani P., Muzi-Falconi M.. Checkpoint mechanisms at the intersection between DNA damage and repair. DNA Repair (Amst). 2009; 8:1055–1067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

