Post-mortem lung tissue: the fossil record of the pathophysiology and immunopathology of severe COVID-19
- PMID: 34871544
- PMCID: PMC8641959
- DOI: 10.1016/S2213-2600(21)00408-2
Post-mortem lung tissue: the fossil record of the pathophysiology and immunopathology of severe COVID-19
Abstract
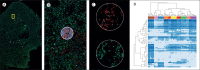
The lungs are the main site that is affected in severe COVID-19, and post-mortem lung tissue provides crucial insights into the pathophysiology of severe disease. From basic histology to state-of-the-art multiparameter digital pathology technologies, post-mortem lung tissue provides snapshots of tissue architecture, and resident and inflammatory cell phenotypes and composition at the time of death. Contrary to early assumptions that COVID-19 in the lungs is a uniform disease, post-mortem findings have established a high degree of disease heterogeneity. Classic diffuse alveolar damage represents just one phenotype, with disease divisible by early and late progression as well as by pathophysiological process. A distinct lung tissue state occurs with secondary infection; extrapulmonary causes of death might also originate from a pathological process in the lungs linked to microthrombosis. This heterogeneity of COVID-19 lung disease must be recognised in the management of patients and in the development of novel treatment strategies.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

