Increased glycolysis affects β-cell function and identity in aging and diabetes
- PMID: 34871777
- PMCID: PMC8732780
- DOI: 10.1016/j.molmet.2021.101414
Increased glycolysis affects β-cell function and identity in aging and diabetes
Abstract
Objective: Age is a risk factor for type 2 diabetes (T2D). We aimed to elucidate whether β-cell glucose metabolism is altered with aging and contributes to T2D.
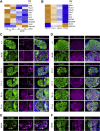
Methods: We used senescence-accelerated mice (SAM), C57BL/6J (B6) mice, and ob/ob mice as aging models. As a diabetes model, we used db/db mice. The glucose responsiveness of insulin secretion and the [U-13C]-glucose metabolic flux were examined in isolated islets. We analyzed the expression of β-cell-specific genes in isolated islets and pancreatic sections as molecular signatures of β-cell identity. β cells defective in the malate-aspartate (MA) shuttle were previously generated from MIN6-K8 cells by the knockout of Got1, a component of the shuttle. We analyzed Got1 KO β cells as a model of increased glycolysis.
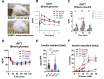
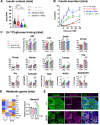
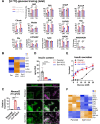
Results: We identified hyperresponsiveness to glucose and compromised cellular identity as dysfunctional phenotypes shared in common between aged and diabetic mouse β cells. We also observed a metabolic commonality between aged and diabetic β cells: hyperactive glycolysis through the increased expression of nicotinamide mononucleotide adenylyl transferase 2 (Nmnat2), a cytosolic nicotinamide adenine dinucleotide (NAD)-synthesizing enzyme. Got1 KO β cells showed increased glycolysis, β-cell dysfunction, and impaired cellular identity, phenocopying aging and diabetes. Using Got1 KO β cells, we show that attenuation of glycolysis or Nmnat2 activity can restore β-cell function and identity.
Conclusions: Our study demonstrates that hyperactive glycolysis is a metabolic signature of aged and diabetic β cells, which may underlie age-related β-cell dysfunction and loss of cellular identity. We suggest Nmnat2 suppression as an approach to counteract age-related T2D.
Keywords: Aging; Diabetes; Glycolysis; Insulin; NAD; β cells.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures


Similar articles
-
Metallothionein 1 negatively regulates glucose-stimulated insulin secretion and is differentially expressed in conditions of beta cell compensation and failure in mice and humans.Diabetologia. 2019 Dec;62(12):2273-2286. doi: 10.1007/s00125-019-05008-3. Epub 2019 Oct 17. Diabetologia. 2019. PMID: 31624901
-
Inhibition of the malate-aspartate shuttle in mouse pancreatic islets abolishes glucagon secretion without affecting insulin secretion.Biochem J. 2015 May 15;468(1):49-63. doi: 10.1042/BJ20140697. Biochem J. 2015. PMID: 25731850 Free PMC article.
-
Overexpression of Gjb4 impairs cell proliferation and insulin secretion in primary islet cells.Mol Metab. 2020 Nov;41:101042. doi: 10.1016/j.molmet.2020.101042. Epub 2020 Jun 18. Mol Metab. 2020. PMID: 32565358 Free PMC article.
-
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells.Front Endocrinol (Lausanne). 2022 Mar 31;13:869414. doi: 10.3389/fendo.2022.869414. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432205 Free PMC article. Review.
-
Does epigenetic dysregulation of pancreatic islets contribute to impaired insulin secretion and type 2 diabetes?Biochem Cell Biol. 2015 Oct;93(5):511-21. doi: 10.1139/bcb-2015-0057. Epub 2015 Aug 4. Biochem Cell Biol. 2015. PMID: 26369706 Review.
Cited by
-
Biomarkers of aging.Sci China Life Sci. 2023 May;66(5):893-1066. doi: 10.1007/s11427-023-2305-0. Epub 2023 Apr 11. Sci China Life Sci. 2023. PMID: 37076725 Free PMC article. Review.
-
The role of GOT1 in cancer metabolism.Front Oncol. 2024 Dec 24;14:1519046. doi: 10.3389/fonc.2024.1519046. eCollection 2024. Front Oncol. 2024. PMID: 39777342 Free PMC article. Review.
-
Monocyte CCL2 signaling possibly contributes to increased asthma susceptibility in type 2 diabetes.Sci Rep. 2025 Mar 28;15(1):10768. doi: 10.1038/s41598-025-95039-1. Sci Rep. 2025. PMID: 40155667 Free PMC article.
-
Pancreatic β-cell failure, clinical implications, and therapeutic strategies in type 2 diabetes.Chin Med J (Engl). 2024 Apr 5;137(7):791-805. doi: 10.1097/CM9.0000000000003034. Epub 2024 Mar 13. Chin Med J (Engl). 2024. PMID: 38479993 Free PMC article. Review.
-
Stem Cell-Derived Islets for Type 2 Diabetes.Int J Mol Sci. 2022 May 4;23(9):5099. doi: 10.3390/ijms23095099. Int J Mol Sci. 2022. PMID: 35563490 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

