Quantile-Dependent Heritability of Glucose, Insulin, Proinsulin, Insulin Resistance, and Glycated Hemoglobin
- PMID: 34872092
- PMCID: PMC8766916
- DOI: 10.1159/000519382
Quantile-Dependent Heritability of Glucose, Insulin, Proinsulin, Insulin Resistance, and Glycated Hemoglobin
Abstract
Background: "Quantile-dependent expressivity" is a dependence of genetic effects on whether the phenotype (e.g., insulin resistance) is high or low relative to its distribution.
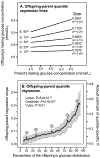
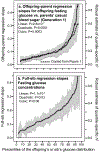
Methods: Quantile-specific offspring-parent regression slopes (βOP) were estimated by quantile regression for fasting glucose concentrations in 6,453 offspring-parent pairs from the Framingham Heart Study.
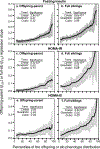
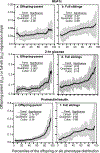
Results: Quantile-specific heritability (h2), estimated by 2βOP/(1 + rspouse), increased 0.0045 ± 0.0007 (p = 8.8 × 10-14) for each 1% increment in the fasting glucose distribution, that is, h2 ± SE were 0.057 ± 0.021, 0.095 ± 0.024, 0.146 ± 0.019, 0.293 ± 0.038, and 0.456 ± 0.061 at the 10th, 25th, 50th, 75th, and 90th percentiles of the fasting glucose distribution, respectively. Significant increases in quantile-specific heritability were also suggested for fasting insulin (p = 1.2 × 10-6), homeostatic model assessment of insulin resistance (HOMA-IR, p = 5.3 × 10-5), insulin/glucose ratio (p = 3.9 × 10-5), proinsulin (p = 1.4 × 10-6), proinsulin/insulin ratio (p = 2.7 × 10-5), and glucose concentrations during a glucose tolerance test (p = 0.001), and their logarithmically transformed values.
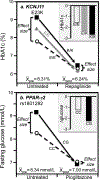
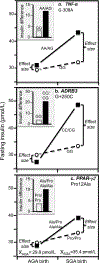
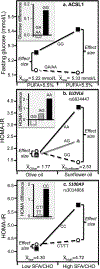
Discussion/conclusion: These findings suggest alternative interpretations to precision medicine and gene-environment interactions, including alternative interpretation of reported synergisms between ACE, ADRB3, PPAR-γ2, and TNF-α polymorphisms and being born small for gestational age on adult insulin resistance (fetal origin theory), and gene-adiposity (APOE, ENPP1, GCKR, IGF2BP2, IL-6, IRS-1, KIAA0280, LEPR, MFHAS1, RETN, TCF7L2), gene-exercise (INS), gene-diet (ACSL1, ELOVL6, IRS-1, PLIN, S100A9), and gene-socioeconomic interactions.
Keywords: Adiposity; Gene environment interaction; Glycemia; Heritability; Insulin.
© 2021 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Figures




Similar articles
-
Spirometric traits show quantile-dependent heritability, which may contribute to their gene-environment interactions with smoking and pollution.PeerJ. 2020 May 15;8:e9145. doi: 10.7717/peerj.9145. eCollection 2020. PeerJ. 2020. PMID: 32461834 Free PMC article.
-
Gene-environment interactions due to quantile-specific heritability of triglyceride and VLDL concentrations.Sci Rep. 2020 Mar 11;10(1):4486. doi: 10.1038/s41598-020-60965-9. Sci Rep. 2020. PMID: 32161301 Free PMC article.
-
Quantile-specific heritability of plasma fibrinogen concentrations.PLoS One. 2022 Jan 7;17(1):e0262395. doi: 10.1371/journal.pone.0262395. eCollection 2022. PLoS One. 2022. PMID: 34995330 Free PMC article.
-
Hyperproinsulinemia in a three-generation Caucasian family due to mutant proinsulin (Arg65-His) not associated with imparied glucose tolerance: the contribution of mutant proinsulin to insulin bioactivity.J Clin Endocrinol Metab. 1996 Apr;81(4):1634-40. doi: 10.1210/jcem.81.4.8636380. J Clin Endocrinol Metab. 1996. PMID: 8636380 Review.
-
Increased Dairy Product Intake Modifies Plasma Glucose Concentrations and Glycated Hemoglobin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Adv Nutr. 2019 Mar 1;10(2):262-279. doi: 10.1093/advances/nmy074. Adv Nutr. 2019. PMID: 30657868 Free PMC article.
Cited by
-
Genetic associations vary across the spectrum of fasting serum insulin: results from the European IDEFICS/I.Family children's cohort.Diabetologia. 2023 Oct;66(10):1914-1924. doi: 10.1007/s00125-023-05957-w. Epub 2023 Jul 7. Diabetologia. 2023. PMID: 37420130 Free PMC article.
-
Quantile-Specific Heritability of Inflammatory and Oxidative Stress Biomarkers Linked to Cardiovascular Disease.J Inflamm Res. 2022 Jan 5;15:85-103. doi: 10.2147/JIR.S347402. eCollection 2022. J Inflamm Res. 2022. PMID: 35023945 Free PMC article.
-
Quantile-specific heritability of 8-isoprostane and the modulating effects of smoking, alcohol, cardiovascular disease and diabetes on 8-isoprostane-gene interactions.Free Radic Biol Med. 2022 Jan;178:262-270. doi: 10.1016/j.freeradbiomed.2021.11.022. Epub 2021 Dec 7. Free Radic Biol Med. 2022. PMID: 34883250 Free PMC article.
References
-
- Falconer DS, Mackay TFC. Introduction to Quantative Genetics. 4th edition. 2004. Pearson Education Limited. London: ISBN 978-81-317-2740-9
-
- Koenker R, Hallock KF. Quantile regression. J Economic Perspectives. 2001;15:143–56. doi.org/10.1257/jep.15.4.143. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

