Neutrophil transit time and localization within the megakaryocyte define morphologically distinct forms of emperipolesis
- PMID: 34872109
- PMCID: PMC9006297
- DOI: 10.1182/bloodadvances.2021005097
Neutrophil transit time and localization within the megakaryocyte define morphologically distinct forms of emperipolesis
Abstract
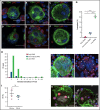
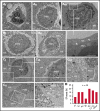
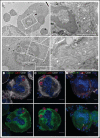
Neutrophils transit through megakaryocytes in a process termed emperipolesis, but it is unknown whether this interaction is a single type of cell-in-cell interaction or a set of distinct processes. Using a murine in vitro model, we characterized emperipolesis by live-cell spinning disk microscopy and electron microscopy. Approximately half of neutrophils exited the megakaryocyte rapidly, typically in 10 minutes or less, displaying ameboid morphology as they passed through the host cell (fast emperipolesis). The remaining neutrophils assumed a sessile morphology, most remaining within the megakaryocyte for at least 60 minutes (slow emperipolesis). These neutrophils typically localized near the megakaryocyte nucleus. By ultrastructural assessment, all internalized neutrophils remained morphologically intact. Most neutrophils resided within emperisomes, but some could be visualized exiting the emperisome to enter the cell cytoplasm. Neutrophils in the cytoplasm assumed close contact with the platelet-forming demarcation membrane system or the perinuclear endoplasmic reticulum. These findings reveal that megakaryocyte emperipolesis reflects at least 2 distinct processes differing in transit time and morphology, fast and slow emperipolesis, suggesting divergent physiologic functions.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures