The Retropepsin-Type Protease APRc as a Novel Ig-Binding Protein and Moonlighting Immune Evasion Factor of Rickettsia
- PMID: 34872352
- PMCID: PMC8649778
- DOI: 10.1128/mBio.03059-21
The Retropepsin-Type Protease APRc as a Novel Ig-Binding Protein and Moonlighting Immune Evasion Factor of Rickettsia
Abstract
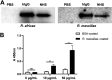
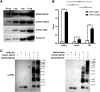
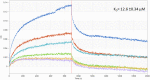
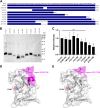
Rickettsiae are obligate intracellular Gram-negative bacteria transmitted by arthropod vectors. Despite their reduced genomes, the function(s) of the majority of rickettsial proteins remains to be uncovered. APRc is a highly conserved retropepsin-type protease, suggested to act as a modulator of other rickettsial surface proteins with a role in adhesion/invasion. However, APRc's function(s) in bacterial pathogenesis and virulence remains unknown. This study demonstrates that APRc targets host serum components, combining nonimmune immunoglobulin (Ig)-binding activity with resistance to complement-mediated killing. We confirmed nonimmune human IgG binding in extracts of different rickettsial species and intact bacteria. Our results revealed that the soluble domain of APRc is capable of binding to human (h), mouse, and rabbit IgG and different classes of human Ig (IgG, IgM, and IgA) in a concentration-dependent manner. APRc-hIgG interaction was confirmed with total hIgG and normal human serum. APRc-hIgG displayed a binding affinity in the micromolar range. We provided evidence of interaction preferentially through the Fab region and confirmed that binding is independent of catalytic activity. Mapping the APRc region responsible for binding revealed the segment between amino acids 157 and 166 as one of the interacting regions. Furthermore, we demonstrated that expression of the full-length protease in Escherichia coli is sufficient to promote resistance to complement-mediated killing and that interaction with IgG contributes to serum resistance. Our findings position APRc as a novel Ig-binding protein and a novel moonlighting immune evasion factor of Rickettsia, contributing to the arsenal of virulence factors utilized by these intracellular pathogens to aid in host colonization. IMPORTANCE Many Rickettsia organisms are pathogenic to humans, causing severe infections, like Rocky Mountain spotted fever and Mediterranean spotted fever. However, immune evasion mechanisms and pathogenicity determinants in rickettsiae are far from being resolved. We provide evidence that the highly conserved rickettsial retropepsin-type protease APRc displays nonimmune immunoglobulin (Ig)-binding activity and participates in serum resistance. APRc emerges then as a novel Ig-binding protein from Gram-negative bacteria and the first to be identified in Rickettsia. Bacterial surface proteins capable of Ig binding are known to be multifunctional and key players in immune evasion. We demonstrate that APRc is also a novel moonlighting protein, exhibiting different actions on serum components and acting as a novel evasin. This work strengthens APRc as a virulence factor in Rickettsia and its significance as a potential therapeutic target. Our findings significantly contribute to a deeper understanding of the virulence strategies used by intracellular pathogens to subvert host immune responses.
Keywords: APRc; Rickettsia; aspartic protease; evasin; immune evasion; nonimmune immunoglobulin-binding; retropepsin; serum resistance.
Figures




Similar articles
-
RC1339/APRc from Rickettsia conorii is a novel aspartic protease with properties of retropepsin-like enzymes.PLoS Pathog. 2014 Aug 21;10(8):e1004324. doi: 10.1371/journal.ppat.1004324. eCollection 2014 Aug. PLoS Pathog. 2014. PMID: 25144529 Free PMC article.
-
Spotted Fever Group Rickettsia Trigger Species-Specific Alterations in Macrophage Proteome Signatures with Different Impacts in Host Innate Inflammatory Responses.Microbiol Spectr. 2021 Dec 22;9(3):e0081421. doi: 10.1128/spectrum.00814-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34935429 Free PMC article.
-
Pathogenic Rickettsia species acquire vitronectin from human serum to promote resistance to complement-mediated killing.Cell Microbiol. 2014 Jun;16(6):849-61. doi: 10.1111/cmi.12243. Epub 2013 Dec 13. Cell Microbiol. 2014. PMID: 24286496 Free PMC article.
-
Pathogenicity and virulence of Rickettsia.Virulence. 2022 Dec;13(1):1752-1771. doi: 10.1080/21505594.2022.2132047. Virulence. 2022. PMID: 36208040 Free PMC article. Review.
-
Pathogenesis of Rickettsial Diseases: Pathogenic and Immune Mechanisms of an Endotheliotropic Infection.Annu Rev Pathol. 2019 Jan 24;14:127-152. doi: 10.1146/annurev-pathmechdis-012418-012800. Epub 2018 Aug 27. Annu Rev Pathol. 2019. PMID: 30148688 Free PMC article. Review.
Cited by
-
Identification of an autotransporter peptidase of Rickettsia rickettsii responsible for maturation of surface exposed autotransporters.PLoS Pathog. 2023 Jul 31;19(7):e1011527. doi: 10.1371/journal.ppat.1011527. eCollection 2023 Jul. PLoS Pathog. 2023. PMID: 37523399 Free PMC article.
-
Secreted retropepsin-like enzymes are essential for stress tolerance and biofilm formation in Pseudomonas aeruginosa.mBio. 2025 Jul 9;16(7):e0087225. doi: 10.1128/mbio.00872-25. Epub 2025 Jun 3. mBio. 2025. PMID: 40459290 Free PMC article.
-
Microbial evasion of the complement system: a continuous and evolving story.Front Immunol. 2024 Jan 4;14:1281096. doi: 10.3389/fimmu.2023.1281096. eCollection 2023. Front Immunol. 2024. PMID: 38239357 Free PMC article. Review.
-
Secreted retropepsin-like enzymes are essential for stress tolerance and biofilm formation in Pseudomonas aeruginosa.bioRxiv [Preprint]. 2025 Apr 6:2025.03.18.643919. doi: 10.1101/2025.03.18.643919. bioRxiv. 2025. Update in: mBio. 2025 Jul 9;16(7):e0087225. doi: 10.1128/mbio.00872-25. PMID: 40166241 Free PMC article. Updated. Preprint.
-
Moonlighting in Rickettsiales: Expanding Virulence Landscape.Trop Med Infect Dis. 2022 Feb 19;7(2):32. doi: 10.3390/tropicalmed7020032. Trop Med Infect Dis. 2022. PMID: 35202227 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
