Evaluation of different platforms for the detection of anti-SARS coronavirus-2 antibodies, Thailand
- PMID: 34872510
- PMCID: PMC8646009
- DOI: 10.1186/s12879-021-06921-y
Evaluation of different platforms for the detection of anti-SARS coronavirus-2 antibodies, Thailand
Abstract
Background: Antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) help determine previous infection in individuals, regardless of whether they are asymptomatic or symptomatic. The detection of antibodies serves several purposes, including supporting other assays for disease diagnosis, conducting seroepidemiological studies, and evaluating vaccines. Many platforms of immunological methods for anti-SARS-CoV-2 antibody detection and their performance require validation.
Methods: This study evaluated the test performance of three autoanalyzer-based assays (Architect IgG, Vitros IgG, and Vitros total Ig) and one manual ELISA (Wantai total Ig) against a microneutralization (microNT) assay on the detection of SARS-CoV-2 antibodies. Furthermore, an indirect immunofluorescence assay verified the discordant results between the microNT and commercial assays. The test sensitivity, specificity, positive predictive value, and negative predictive value were determined based on four groups of 1005 serum samples: 102 COVID-19 prepandemic sera, 45 anti-SARS-CoV-2 positive sera, 366 sera of people at risk, and 492 sera of citizens returning from countries with a high prevalence of infection.
Results: The analyses as a whole showed that the performance of these commercial assays was comparable. Each group was also analysed separately to gain further insight into test performance. The Architect did not detect two positive sera of people at risk (prevalence of infection 0.55%). The other methods correctly identified these two positive sera but yielded varying false-positive results. The group of returning travellers with an infection rate of 28.3% (139 of 492) better differentiated the test performance of individual assays.
Conclusions: High-throughput Architect and Vitros autoanalyzers appear appropriate for working on large sample sizes in countries that can afford the cost. The Wantai ELISA, while requiring more individual time and technical skill, may provide reliable results at a lower cost. The selection of assays will depend on the laboratory facilities and feasibility.
Keywords: Antibody detection; Chemiluminescence assay; ELISA; Microneutralization assay; SARS-coronavirus-2.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
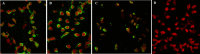
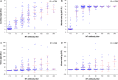
References
-
- Premkumar L, Segovia-Chumbez B, Jadi R, Martinez DR, Raut R, Markmann A, et al. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5(48):eabc8413. doi: 10.1126/sciimmunol.abc8413. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. Interim guidelines for COVID-19 antibody testing in clinical and public health settings. 2021. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-g.... Updated 17 Mar 2021. Accessed 15 June 2021.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

