The biology of pancreatic cancer morphology
- PMID: 34872751
- PMCID: PMC8891077
- DOI: 10.1016/j.pathol.2021.09.012
The biology of pancreatic cancer morphology
Abstract
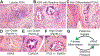
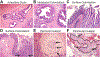
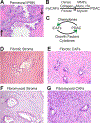
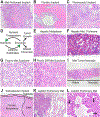
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal of all human malignancies. PDAC precursor lesions, invasive primary PDAC, and metastatic PDAC each display distinct morphologies that reflect unique biology. This 'biomorphology' is determined by a complex neoplastic history of clonal phylogenetic relationships, geographic locations, external environmental exposures, intrinsic metabolic demands, and tissue migration patterns. Understanding the biomorphological evolution of PDAC progression is not only of academic interest but also of great practical value. Applying this knowledge to surgical pathology practice facilitates the correct diagnosis on routine H&E stains without additional ancillary studies in most cases. Here I provide a concise overview of the entire biomorphological spectrum of PDAC progression beginning with initial neoplastic transformation and ending in terminal distant metastasis. Most biopsy and resection specimens are currently obtained prior to treatment. As such, our understanding of untreated PDAC biomorphology is mature. The biomorphology of treated PDAC is less defined but will assume greater importance as the frequency of neoadjuvant therapy increases. Although this overview is slanted towards pathology, it is written so that pathologists, clinicians, and scientists alike might find it instructive for their respective disciplines.
Keywords: Pancreatic cancer; biology; morphology; pancreatic ductal adenocarcinoma; pathology.
Copyright © 2021 Royal College of Pathologists of Australasia. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Aspartate β-hydroxylase promotes pancreatic ductal adenocarcinoma metastasis through activation of SRC signaling pathway.J Hematol Oncol. 2019 Dec 30;12(1):144. doi: 10.1186/s13045-019-0837-z. J Hematol Oncol. 2019. PMID: 31888763 Free PMC article.
-
miRNA and Gene Expression in Pancreatic Ductal Adenocarcinoma.Am J Pathol. 2019 Jan;189(1):58-70. doi: 10.1016/j.ajpath.2018.10.005. Am J Pathol. 2019. PMID: 30558723 Free PMC article. Review.
-
Non-coding RNA biomarkers in pancreatic ductal adenocarcinoma.Semin Cancer Biol. 2021 Oct;75:153-168. doi: 10.1016/j.semcancer.2020.10.001. Epub 2020 Oct 10. Semin Cancer Biol. 2021. PMID: 33049362 Free PMC article. Review.
-
SIRT 1 Overexpression is Associated with Metastasis of Pancreatic Ductal Adenocarcinoma (PDAC) and Promotes Migration and Growth of PDAC Cells.Med Sci Monit. 2016 May 12;22:1593-600. doi: 10.12659/msm.896697. Med Sci Monit. 2016. PMID: 27170223 Free PMC article.
-
Emerging Evidence for the Clinical Relevance of Pancreatic Cancer Exosomes.Pancreas. 2019 Jan;48(1):1-8. doi: 10.1097/MPA.0000000000001203. Pancreas. 2019. PMID: 30531240 Review.
Cited by
-
Prognostic Impact of Phenotypic and Genetic Features of Pancreatic Malignancies.Life (Basel). 2025 Apr 11;15(4):635. doi: 10.3390/life15040635. Life (Basel). 2025. PMID: 40283189 Free PMC article. Review.
-
The Desmoplastic Stroma of Pancreatic Cancer: Multilayered Levels of Heterogeneity, Clinical Significance, and Therapeutic Opportunities.Cancers (Basel). 2022 Jul 5;14(13):3293. doi: 10.3390/cancers14133293. Cancers (Basel). 2022. PMID: 35805064 Free PMC article. Review.
-
Senescence program and its reprogramming in pancreatic premalignancy.Cell Death Dis. 2023 Aug 17;14(8):528. doi: 10.1038/s41419-023-06040-3. Cell Death Dis. 2023. PMID: 37591827 Free PMC article. Review.
-
Pancreatic cancer epigenetics: adaptive metabolism reprograms starving primary tumors for widespread metastatic outgrowth.Cancer Metastasis Rev. 2023 Jun;42(2):389-407. doi: 10.1007/s10555-023-10116-z. Epub 2023 Jun 15. Cancer Metastasis Rev. 2023. PMID: 37316634 Free PMC article. Review.
-
The Significance of Neuropilins in Gastrointestinal Cancers.Int J Mol Sci. 2025 May 21;26(10):4937. doi: 10.3390/ijms26104937. Int J Mol Sci. 2025. PMID: 40430075 Free PMC article. Review.
References
-
- Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res 2000; 6: 2969–72. - PubMed
-
- Guerra C, Schuhmacher AJ, Cañamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 2007; 11: 291–302. - PubMed
-
- Hayashi A, Hong J, Iacobuzio-Donahue CA. The pancreatic cancer genome revisited. Nat Rev Gastroenterol Hepatol 2021; 18: 469–81. - PubMed
-
- Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst 1997; 89: 442–6. - PubMed
-
- Hruban RH, van Mansfeld AD, Offerhaus GJ, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol 1993; 143: 545–54. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

