Lunatic Fringe-GFP Marks Lamina-Specific Astrocytes That Regulate Sensory Processing
- PMID: 34872929
- PMCID: PMC8805626
- DOI: 10.1523/JNEUROSCI.1392-21.2021
Lunatic Fringe-GFP Marks Lamina-Specific Astrocytes That Regulate Sensory Processing
Abstract
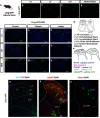
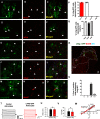
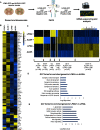
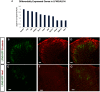
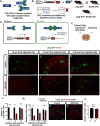
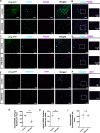
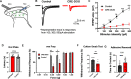
Astrocytes are the most abundant glial cell in the brain and perform a wide range of tasks that support neuronal function and circuit activities. There is emerging evidence that astrocytes exhibit molecular and cellular heterogeneity; however, whether distinct subpopulations perform these diverse roles remains poorly defined. Here we show that the Lunatic Fringe-GFP (Lfng-GFP) bacteria artificial chromosome mouse line from both sexes specifically labels astrocyte populations within lamina III and IV of the dorsal spinal cord. Transcriptional profiling of Lfng-GFP+ astrocytes revealed unique molecular profiles, featuring an enriched expression of Notch- and Wnt- pathway components. Leveraging CRE-DOG viral tools, we ablated Lfng-GFP+ astrocytes, which decreased neuronal activity in lamina III and IV and impaired mechanosensation associated with light touch. Together, our findings identify Lfng-GFP+ astrocytes as a unique subpopulation that occupies a distinct anatomic location in the spinal cord and directly contributes to neuronal function and sensory responses.SIGNIFICANCE STATEMENT Astrocytes are the most abundant glial cell in the CNS, and their interactions with neurons are essential for brain function. However, understanding the functional diversity of astrocytes has been hindered because of the lack of reporters that mark subpopulations and genetic tools for accessing them. We discovered that the Lfng-GFP reporter mouse labels a laminae-specific subpopulation of astrocytes in the dorsal spinal cord and that ablation of these astrocytes reduces glutamatergic synapses. Further analysis revealed that these astrocytes have a role in maintaining sensory-processing circuity related to light touch.
Keywords: astrocyte; circuit activities; glia; sensory processing; spinal cord.
Copyright © 2022 the authors.
Figures
References
-
- Basch ML, Brown RM, Jen H-I, Semerci F, Depreux F, Edlund R, Zhang H, Norton CR, Gridley T, Cole SE, Doetzlhofer A, Maletic-Savatic M, Segil N, Groves AK (2016) Fine-tuning of Notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates. eLife 5:e19921. 10.7554/eLife.19921 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
