Dermatomyositis: Muscle Pathology According to Antibody Subtypes
- PMID: 34873015
- PMCID: PMC8865893
- DOI: 10.1212/WNL.0000000000013176
Dermatomyositis: Muscle Pathology According to Antibody Subtypes
Abstract
Background and objectives: Discoveries of dermatomyositis-specific antibodies (DMSAs) in patients with dermatomyositis raised awareness of various myopathologic features among antibody subtypes. However, only perifascicular atrophy and perifascicular myxovirus resistant protein A (MxA) overexpression were officially included as definitive pathologic criteria for dermatomyositis classification. We aimed to demonstrate myopathologic features in MxA-positive dermatomyositis to determine characteristic myopathologic features in different DMSA subtypes.
Methods: We performed a retrospective pathology review of muscle biopsies of patients with dermatomyositis diagnosed between January 2009 and December 2020 in a tertiary laboratory for muscle diseases. We included all muscle biopsies with sarcoplasmic expression for MxA and seropositivity for DMSAs. MxA-positive muscle biopsies that tested negative for all DMSAs were included as seronegative dermatomyositis. We evaluated histologic features stratified according to 4 pathology domains (muscle fiber, inflammatory, vascular, and connective tissue) and histologic features of interest by histochemistry, enzyme histochemistry, and immunohistochemical study commonly used in the diagnosis of inflammatory myopathy. We performed ultrastructural studies of 54 available specimens.
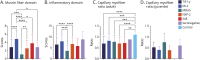
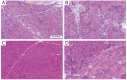
Results: A total of 256 patients were included. Of these, 249 patients were positive for 1 of the 5 DMSAs (seropositive patients: 87 anti-transcription intermediary factor 1-γ [TIF1-γ], 40 anti-complex nucleosome remodeling histone deacetylase [Mi-2], 29 anti-melanoma differentiation gene 5 [MDA5], 83 anti-nuclear matrix protein 2 [NXP-2], and 10 anti-small ubiquitin-like modifier-activating enzyme [SAE] dermatomyositis) and 7 patients were negative for all 5 DMSAs (seronegative patients). Characteristic myopathologic features in each DMSA subtype were as follows: anti-TIF1-γ with vacuolated/punched out fibers (64.7%; p < 0.001) and perifascicular enhancement in HLA-ABC stain (75.9%; p < 0.001); anti-Mi-2 with prominent muscle fiber damage (score 4.9 ± 2.1; p < 0.001), inflammatory cell infiltration (score 8.0 ± 3.0; p = 0.002), perifascicular atrophy (67.5%; p = 0.02), perifascicular necrosis (52.5%; p < 0.001), increased perimysial alkaline phosphatase activity (70.0%; p < 0.001), central necrotic peripheral regenerating fibers (45.0%; p = 0.002), and sarcolemmal membrane attack complex deposition (67.5%; p < 0.001); anti-MDA5 with scattered/diffuse staining pattern of MxA (65.5%; p < 0.001) with less muscle pathology and inflammatory features; anti-NXP-2 with microinfarction (26.5%; p < 0.001); and anti-SAE and seronegative dermatomyositis with HLA-DR expression (50.0%; p = 0.02 and 57.1%; p = 0.02, respectively).
Discussion: We describe a comprehensive serologic-pathologic correlation of dermatomyositis primarily using MxA expression as an inclusion criterion. In our study, DMSAs were associated with distinctive myopathologic features suggesting different underlying pathobiologic mechanisms in each subtype.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
-
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292(7):344-347. - PubMed
-
- Mammen AL, Allenbach Y, Stenzel W, Benveniste O. ENMC 239th Workshop Study Group. 239th ENMC international workshop: classification of dermatomyositis, Amsterdam, The Netherlands, 14-16 December 2018. Neuromuscul Disord. 2020;30(1):70-92. - PubMed
-
- Tanboon J, Uruha A, Stenzel W, Nishino I. Where are we moving in the classification of idiopathic inflammatory myopathies? Curr Opin Neurol. 2020;33(5):590-603. - PubMed
-
- Hida A, Yamashita T, Hosono Y, et al. Anti-TIF1-γ antibody and cancer-associated myositis: a clinicohistopathologic study. Neurology. 2016;87(3):299-308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
