Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients
- PMID: 34873151
- PMCID: PMC8646019
- DOI: 10.1038/s41392-021-00835-6
Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients
Abstract
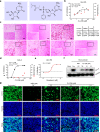
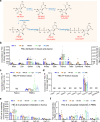
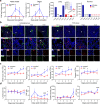
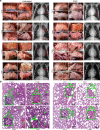
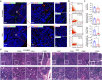
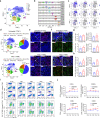
Azvudine (FNC) is a nucleoside analog that inhibits HIV-1 RNA-dependent RNA polymerase (RdRp). Recently, we discovered FNC an agent against SARS-CoV-2, and have taken it into Phase III trial for COVID-19 patients. FNC monophosphate analog inhibited SARS-CoV-2 and HCoV-OC43 coronavirus with an EC50 between 1.2 and 4.3 μM, depending on viruses or cells, and selective index (SI) in 15-83 range. Oral administration of FNC in rats revealed a substantial thymus-homing feature, with FNC triphosphate (the active form) concentrated in the thymus and peripheral blood mononuclear cells (PBMC). Treating SARS-CoV-2 infected rhesus macaques with FNC (0.07 mg/kg, qd, orally) reduced viral load, recuperated the thymus, improved lymphocyte profiles, alleviated inflammation and organ damage, and lessened ground-glass opacities in chest X-ray. Single-cell sequencing suggested the promotion of thymus function by FNC. A randomized, single-arm clinical trial of FNC on compassionate use (n = 31) showed that oral FNC (5 mg, qd) cured all COVID-19 patients, with 100% viral ribonucleic acid negative conversion in 3.29 ± 2.22 days (range: 1-9 days) and 100% hospital discharge rate in 9.00 ± 4.93 days (range: 2-25 days). The side-effect of FNC is minor and transient dizziness and nausea in 16.12% (5/31) patients. Thus, FNC might cure COVID-19 through its anti-SARS-CoV-2 activity concentrated in the thymus, followed by promoted immunity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

