Oligodendrocytes depend on MCL-1 to prevent spontaneous apoptosis and white matter degeneration
- PMID: 34873168
- PMCID: PMC8648801
- DOI: 10.1038/s41419-021-04422-z
Oligodendrocytes depend on MCL-1 to prevent spontaneous apoptosis and white matter degeneration
Abstract
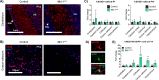
Neurologic disorders often disproportionately affect specific brain regions, and different apoptotic mechanisms may contribute to white matter pathology in leukodystrophies or gray matter pathology in poliodystrophies. We previously showed that neural progenitors that generate cerebellar gray matter depend on the anti-apoptotic protein BCL-xL. Conditional deletion of Bcl-xL in these progenitors produces spontaneous apoptosis and cerebellar hypoplasia, while similar conditional deletion of Mcl-1 produces no phenotype. Here we show that, in contrast, postnatal oligodendrocytes depend on MCL-1. We found that brain-wide Mcl-1 deletion caused apoptosis specifically in mature oligodendrocytes while sparing astrocytes and oligodendrocyte precursors, resulting in impaired myelination and progressive white matter degeneration. Disabling apoptosis through co-deletion of Bax or Bak rescued white matter degeneration, implicating the intrinsic apoptotic pathway in Mcl-1-dependence. Bax and Bak co-deletions rescued different aspects of the Mcl-1-deleted phenotype, demonstrating their discrete roles in white matter stability. MCL-1 protein abundance was reduced in eif2b5-mutant mouse model of the leukodystrophy vanishing white matter disease (VWMD), suggesting the potential for MCL-1 deficiency to contribute to clinical neurologic disease. Our data show that oligodendrocytes require MCL-1 to suppress apoptosis, implicate MCL-1 deficiency in white matter pathology, and suggest apoptosis inhibition as a leukodystrophy therapy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
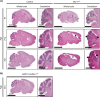

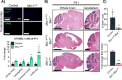
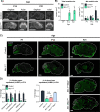
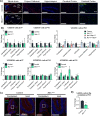

Similar articles
-
Prosurvival Bcl-2 family members affect autophagy only indirectly, by inhibiting Bax and Bak.Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):8512-7. doi: 10.1073/pnas.1406425111. Epub 2014 May 27. Proc Natl Acad Sci U S A. 2014. PMID: 24912196 Free PMC article.
-
Antiapoptotic Bcl-2 family proteins BCL-xL and MCL-1 integrate neural progenitor survival and proliferation during postnatal cerebellar neurogenesis.Cell Death Differ. 2021 May;28(5):1579-1592. doi: 10.1038/s41418-020-00687-7. Epub 2020 Dec 8. Cell Death Differ. 2021. PMID: 33293647 Free PMC article.
-
The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner.J Biol Chem. 2011 Mar 18;286(11):9382-92. doi: 10.1074/jbc.M110.203638. Epub 2010 Dec 9. J Biol Chem. 2011. PMID: 21148306 Free PMC article.
-
Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane.Genes Dev. 2016 Apr 15;30(8):973-88. doi: 10.1101/gad.276725.115. Epub 2016 Apr 7. Genes Dev. 2016. PMID: 27056669 Free PMC article.
-
Neuroglial Pathophysiology of Leukodystrophies.Adv Neurobiol. 2025;43:257-279. doi: 10.1007/978-3-031-87919-7_10. Adv Neurobiol. 2025. PMID: 40500501 Review.
Cited by
-
Emerging mitochondrial-mediated mechanisms involved in oligodendrocyte development.J Neurosci Res. 2023 Mar;101(3):354-366. doi: 10.1002/jnr.25151. Epub 2022 Dec 3. J Neurosci Res. 2023. PMID: 36461887 Free PMC article. Review.
-
MCL-1 regulates cellular transitions during oligodendrocyte development.bioRxiv [Preprint]. 2024 Dec 21:2024.12.20.629796. doi: 10.1101/2024.12.20.629796. bioRxiv. 2024. PMID: 39763750 Free PMC article. Preprint.
-
MCL‑1 safeguards activated hair follicle stem cells to enable adult hair regeneration.Nat Commun. 2025 Mar 22;16(1):2829. doi: 10.1038/s41467-025-58150-5. Nat Commun. 2025. PMID: 40121237 Free PMC article.
-
Neonatal Behavioral Screen for Mouse Models of Neurodevelopmental Disorders.Methods Mol Biol. 2023;2583:159-173. doi: 10.1007/978-1-0716-2752-5_14. Methods Mol Biol. 2023. PMID: 36418733
-
MAB21L1 promotes survival of lens epithelial cells through control of αB-crystallin and ATR/CHK1/p53 pathway.Aging (Albany NY). 2022 Aug 10;14(15):6128-6148. doi: 10.18632/aging.204203. Epub 2022 Aug 10. Aging (Albany NY). 2022. PMID: 35951367 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- U54HD079124/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R01NS102627/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R35 GM128915/GM/NIGMS NIH HHS/United States
- T32CA071341/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- U54 HD079124/HD/NICHD NIH HHS/United States
- R01 NS106227/NS/NINDS NIH HHS/United States
- R01NS088219/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- P50 HD103573/HD/NICHD NIH HHS/United States
- R01 NS102627/NS/NINDS NIH HHS/United States
- R21 CA227483/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

