Nickel-catalyzed electrochemical carboxylation of unactivated aryl and alkyl halides with CO2
- PMID: 34873172
- PMCID: PMC8648755
- DOI: 10.1038/s41467-021-27437-8
Nickel-catalyzed electrochemical carboxylation of unactivated aryl and alkyl halides with CO2
Abstract
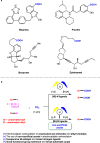
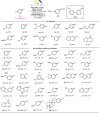
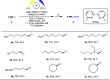
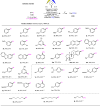
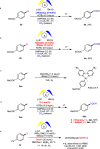
Electrochemical catalytic reductive cross couplings are powerful and sustainable methods to construct C-C bonds by using electron as the clean reductant. However, activated substrates are used in most cases. Herein, we report a general and practical electro-reductive Ni-catalytic system, realizing the electrocatalytic carboxylation of unactivated aryl chlorides and alkyl bromides with CO2. A variety of unactivated aryl bromides, iodides and sulfonates can also undergo such a reaction smoothly. Notably, we also realize the catalytic electrochemical carboxylation of aryl (pseudo)halides with CO2 avoiding the use of sacrificial electrodes. Moreover, this sustainable and economic strategy with electron as the clean reductant features mild conditions, inexpensive catalyst, safe and cheap electrodes, good functional group tolerance and broad substrate scope. Mechanistic investigations indicate that the reaction might proceed via oxidative addition of aryl halides to Ni(0) complex, the reduction of aryl-Ni(II) adduct to the Ni(I) species and following carboxylation with CO2.
© 2021. The Author(s).
Conflict of interest statement
The authors declare the following competing financial interest(s): A Chinese Patent on this work has been authorized with the number (202010244592.7). The authors declare no other competing interests.
Figures



References
-
- Krische M. J. Metal catalyzed reductive C—C bond formation (Springer, 2007).
-
- Liu J, Ye Y, Sessler JL, Gong H. Cross-electrophile couplings of activated and sterically hindered halides and alcohol derivatives. Acc. Chem. Res. 2020;53:1833–1845. - PubMed
-
- Xia Q, Dong J, Song H, Wang Q. Visible-Light photocatalysis of the ketyl radical coupling reaction. Chem. Eur. J. 2019;25:2949–2961. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

