Decision aid and cost compensation influence uptake of PSA-based early detection without affecting decisional conflict: a cluster randomised trial
- PMID: 34873188
- PMCID: PMC8648904
- DOI: 10.1038/s41598-021-02696-z
Decision aid and cost compensation influence uptake of PSA-based early detection without affecting decisional conflict: a cluster randomised trial
Abstract
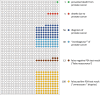
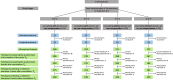
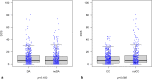
International guidelines recommend to inform men about the benefits and harms of prostate specific antigen (PSA) based early detection of prostate cancer. This study investigates the influence of a transactional decision aid (DA) or cost compensation (CC) for a PSA test on the decisional behaviour of men. Prospective, cluster-randomised trial to compare two interventions in a 2 × 2 factorial design: DA versus counselling as usual, and CC versus noCC for PSA-testing. 90 cluster-randomised physicians in the administrative district of Muenster, Germany recruited 962 participants aged 55-69 yrs. in 2018. Primary endpoint: the influence of the DA and CC on the decisional conflict. Secondary endpoints: factors which altered the involvement of the men regarding their decision to take a PSA-test. The primary endpoint was analysed by a multivariate regression model. The choice to take the PSA test was increased by CC and reduced by the DA, the latter also reduced PSA uptake in men who were offered CC. The DA led to an increase of the median knowledge about early detection, changed willingness to perform a PSA test without increasing the level of shared decision, giving participants a stronger feeling of having made the decision by themselves. The DA did not alter the decisional conflict, as it was very low in all study groups. DA reduced and CC increased the PSA uptake. The DA seemed to have a greater impact on the participants than CC, as it led to fewer PSA tests even if CC was granted.Trial registration: German Clinical Trial Register (Deutsches Register Klinischer Studien DRKS00007687). Registered: 06/05/2015. https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00007687 .
© 2021. The Author(s).
Conflict of interest statement
N.D.B. is co-chairman of the Association for Patient-orientated Communication (GPZK), a non-profit organisation distributing decision-support software, among these the PSA decision aid investigated in this study. He does not obtain a salary or other personal income from GPZK. A.Se reports grants from German Cancer Aid, during the conduct of the study; other from Myriad, from Philips Healthcare, from Proteomedix, personal fees from Janssen, personal fees from Ipsen, outside the submitted work. In addition A.Se has a patent Characterization of primary tumors (039PCT0735) issued. D.T. reports personal fees from Novartis, outside the submitted work. M.B., A.Si, K.S., K.Kr, C.B., K.Ku, C.C.A., P.M., R.J., M.A.K., J.G., C.T., R.B., M.J.R., C.H.B and H.W.H declare no conflicts of interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

