Flavivirus infections induce a Golgi stress response in vertebrate and mosquito cells
- PMID: 34873243
- PMCID: PMC8648732
- DOI: 10.1038/s41598-021-02929-1
Flavivirus infections induce a Golgi stress response in vertebrate and mosquito cells
Abstract
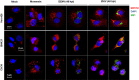
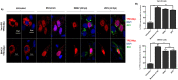
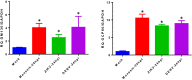
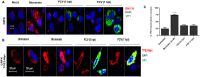
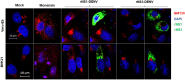
The stress of the Golgi apparatus is an autoregulatory mechanism that is induced to compensate for greater demand in the Golgi functions. No examples of Golgi stress responses due to physiological stimuli are known. Furthermore, the impact on this organelle of viral infections that occupy the vesicular transport during replication is unknown. In this work, we evaluated if a Golgi stress response is triggered during dengue and Zika viruses replication, two flaviviruses whose replicative cycle is heavily involved with the Golgi complex, in vertebrate and mosquito cells. Using GM-130 as a Golgi marker, and treatment with monensin as a positive control for the induction of the Golgi stress response, a significant expansion of the Golgi cisternae was observed in BHK-21, Vero E6 and mosquito cells infected with either virus. Activation of the TFE3 pathway was observed in the infected cells as indicated by the translocation from the cytoplasm to the nucleus of TFE3 and increased expression of pathway targeted genes. Of note, no sign of activation of the stress response was observed in CRFK cells infected with Feline Calicivirus (FCV), a virus released by cell lysis, not requiring vesicular transport. Finally, dilatation of the Golgi complex and translocation of TFE3 was observed in vertebrate cells expressing dengue and Zika viruses NS1, but not NS3. These results indicated that infections by dengue and Zika viruses induce a Golgi stress response in vertebrate and mosquito cells due to the increased demand on the Golgi complex imposed by virion and NS1 processing and secretion.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

