Temperature responsive chromatography for therapeutic drug monitoring with an aqueous mobile phase
- PMID: 34873248
- PMCID: PMC8648775
- DOI: 10.1038/s41598-021-02998-2
Temperature responsive chromatography for therapeutic drug monitoring with an aqueous mobile phase
Abstract
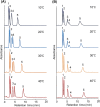
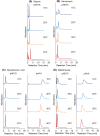
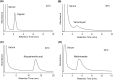
Therapeutic drug monitoring is a key technology for effective pharmacological treatment. In the present study, a temperature-responsive chromatography column was developed for safe and simple therapeutic drug monitoring without the use of organic solvents. Poly(N-isopropylacrylamide) (PNIPAAm) hydrogel-modified silica beads were prepared via a condensation reaction and radical polymerization. The temperature-dependent elution behavior of the drugs was observed using a PNIPAAm-modified silica-bead packed column and an all-aqueous mobile phase. Sharp peaks with reproducible retention times were observed at temperatures of 30 °C or 40 °C because the PNIPAAm hydrogel on the silica beads shrinks at these temperatures, limiting drug diffusion into the PNIPAAm hydrogel layer. The elution behavior of the sample from the prepared column was examined using a mixture of serum and model drugs. The serum and drugs were separated on the column at 30 °C or 40 °C, and the concentration of the eluted drug was obtained using the calibration curve. The results show that the prepared chromatography column would be useful for therapeutic drug monitoring because the drug concentration in serum can be measured without using organic solvents in the mobile phase and without any need for sample preparation.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources

