A nomogram for predicting probability of low risk of MammaPrint results in women with clinically high-risk breast cancer
- PMID: 34873249
- PMCID: PMC8648770
- DOI: 10.1038/s41598-021-02992-8
A nomogram for predicting probability of low risk of MammaPrint results in women with clinically high-risk breast cancer
Abstract
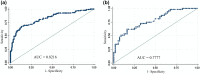
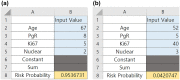
We aimed to develop a prediction MammaPrint (MMP) genomic risk assessment nomogram model for hormone-receptor positive (HR+) and human epidermal growth factor receptor-2 negative (HER2-) breast cancer and minimal axillary burden (N0-1) tumors using clinicopathological factors of patients who underwent an MMP test for decision making regarding adjuvant chemotherapy. A total of 409 T1-3 N0-1 M0 HR + and HER2- breast cancer patients whose MMP genomic risk results and clinicopathological factors were available from 2017 to 2020 were analyzed. With randomly selected 306 patients, we developed a nomogram for predicting a low-risk subgroup of MMP results and externally validated with remaining patients (n = 103). Multivariate analysis revealed that the age at diagnosis, progesterone receptor (PR) score, nuclear grade, and Ki-67 were significantly associated with MMP risk results. We developed an MMP low-risk predictive nomogram. With a cut off value at 5% and 95% probability of low-risk MMP, the nomogram accurately predicted the results with 100% positive predictive value (PPV) and negative predictive value respectively. When applied to cut-off value at 35%, the specificity and PPV was 95% and 86% respectively. The area under the receiver operating characteristic curve was 0.82 (95% confidence interval [CI] 0.77 to 0.87). When applied to the validation group, the nomogram was accurate with an area under the curve of 0.77 (95% CI 0.68 to 0.86). Our nomogram, which incorporates four traditional prognostic factors, i.e., age, PR, nuclear grade, and Ki-67, could predict the probability of obtaining a low MMP risk in a cohort of high clinical risk patients. This nomogram can aid the prompt selection of patients who does not need additional MMP testing.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Nice.org.uk, National Institute for Health Care and Excellence. Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer. Diagnostic Guidance DG34. https://www.nice.org.uk/guidance/dg34/resources/tumour-profiling-tests-t... (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

