Assessment of behavioral, morphological and electrophysiological changes in prenatal and postnatal valproate induced rat models of autism spectrum disorder
- PMID: 34873263
- PMCID: PMC8648736
- DOI: 10.1038/s41598-021-02994-6
Assessment of behavioral, morphological and electrophysiological changes in prenatal and postnatal valproate induced rat models of autism spectrum disorder
Abstract
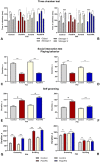
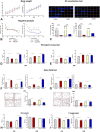
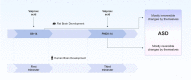
Autism spectrum disorders (ASD) are neurodevelopmental disorders, that are characterized by core symptoms, such as alterations of social communication and restrictive or repetitive behavior. The etiology and pathophysiology of disease is still unknown, however, there is a strong interaction between genetic and environmental factors. An intriguing point in autism research is identification the vulnerable time periods of brain development that lack compensatory homeostatic corrections. Valproic acid (VPA) is an antiepileptic drug with a pronounced teratogenic effect associated with a high risk of ASD, and its administration to rats during the gestation is used for autism modeling. It has been hypothesized that valproate induced damage and functional alterations of autism target structures may occur and evolve during early postnatal life. Here, we used prenatal and postnatal administrations of VPA to investigate the main behavioral features which are associated with autism spectrum disorders core symptoms were tested in early juvenile and adult rats. Neuroanatomical lesion of autism target structures and electrophysiological studies in specific neural circuits. Our results showed that prenatal and early postnatal administration of valproate led to the behavioral alterations that were similar to ASD. Postnatally treated group showed tendency to normalize in adulthood. We found pronounced structural changes in the brain target regions of prenatally VPA-treated groups, and an absence of abnormalities in postnatally VPA-treated groups, which confirmed the different severity of VPA across different stages of brain development. The results of this study clearly show time dependent effect of VPA on neurodevelopment, which might be explained by temporal differences of brain regions' development process. Presumably, postnatal administration of valproate leads to the dysfunction of synaptic networks that is recovered during the lifespan, due to the brain plasticity and compensatory ability of circuit refinement. Therefore, investigations of compensatory homeostatic mechanisms activated after VPA administration and directed to eliminate the defects in postnatal brain, may elucidate strategies to improve the course of disease.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Home | APA DSM-5. http://www.dsm5.org/Pages/Default.aspx.
-
- Yenkoyan K, Grigoryan A, Fereshetyan K, Yepremyan D. Advances in understanding the pathophysiology of autism spectrum disorders. Behav. Brain Res. 2017;331:92–101. - PubMed

