Potential long-term effects of SARS-CoV-2 infection on the pulmonary vasculature: a global perspective
- PMID: 34873286
- PMCID: PMC8647069
- DOI: 10.1038/s41569-021-00640-2
Potential long-term effects of SARS-CoV-2 infection on the pulmonary vasculature: a global perspective
Abstract
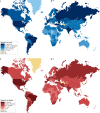
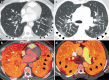
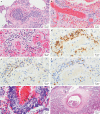
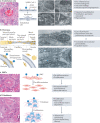
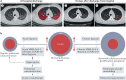
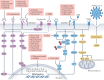
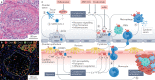
The lungs are the primary target of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with severe hypoxia being the cause of death in the most critical cases. Coronavirus disease 2019 (COVID-19) is extremely heterogeneous in terms of severity, clinical phenotype and, importantly, global distribution. Although the majority of affected patients recover from the acute infection, many continue to suffer from late sequelae affecting various organs, including the lungs. The role of the pulmonary vascular system during the acute and chronic stages of COVID-19 has not been adequately studied. A thorough understanding of the origins and dynamic behaviour of the SARS-CoV-2 virus and the potential causes of heterogeneity in COVID-19 is essential for anticipating and treating the disease, in both the acute and the chronic stages, including the development of chronic pulmonary hypertension. Both COVID-19 and chronic pulmonary hypertension have assumed global dimensions, with potential complex interactions. In this Review, we present an update on the origins and behaviour of the SARS-CoV-2 virus and discuss the potential causes of the heterogeneity of COVID-19. In addition, we summarize the pathobiology of COVID-19, with an emphasis on the role of the pulmonary vasculature, both in the acute stage and in terms of the potential for developing chronic pulmonary hypertension. We hope that the information presented in this Review will help in the development of strategies for the prevention and treatment of the continuing COVID-19 pandemic.
© 2021. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboardhttps://covid19.who.int/ (2021).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

