Species- and site-specific genome editing in complex bacterial communities
- PMID: 34873292
- PMCID: PMC9261505
- DOI: 10.1038/s41564-021-01014-7
Species- and site-specific genome editing in complex bacterial communities
Abstract
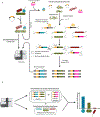
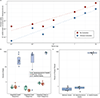
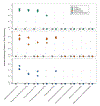
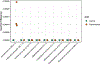
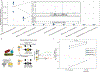
Understanding microbial gene functions relies on the application of experimental genetics in cultured microorganisms. However, the vast majority of bacteria and archaea remain uncultured, precluding the application of traditional genetic methods to these organisms and their interactions. Here, we characterize and validate a generalizable strategy for editing the genomes of specific organisms in microbial communities. We apply environmental transformation sequencing (ET-seq), in which nontargeted transposon insertions are mapped and quantified following delivery to a microbial community, to identify genetically tractable constituents. Next, DNA-editing all-in-one RNA-guided CRISPR-Cas transposase (DART) systems for targeted DNA insertion into organisms identified as tractable by ET-seq are used to enable organism- and locus-specific genetic manipulation in a community context. Using a combination of ET-seq and DART in soil and infant gut microbiota, we conduct species- and site-specific edits in several bacteria, measure gene fitness in a nonmodel bacterium and enrich targeted species. These tools enable editing of microbial communities for understanding and control.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests
The Regents of the University of California have patents pending related to this work on which B.E.R., S.D., B.F.C., A.M.D., J.F.B., and J.A.D. are inventors. J.A.D. is a co-founder of Caribou Biosciences, Editas Medicine, Intellia Therapeutics, Scribe Therapeutics and Mammoth Biosciences, a scientific advisory board member of Caribou Biosciences, Intellia Therapeutics, eFFECTOR Therapeutics, Scribe Therapeutics, Synthego, Mammoth Biosciences and Inari, and is a Director at Johnson & Johnson and has sponsored research projects by Biogen, Roche and Pfizer. J.F.B. is a founder of Metagenomi. R.B. is a shareholder of Caribou Biosciences, Intellia Therapeutics, Locus Biosciences, Inari, TreeCo, and Ancilia Biosciences.
Figures








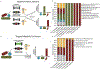

Comment in
-
DART takes aim at community editing.Nat Microbiol. 2022 Jan;7(1):8-9. doi: 10.1038/s41564-021-01017-4. Nat Microbiol. 2022. PMID: 34873294 No abstract available.
-
CRISPR editing within microbial communities.Nat Rev Genet. 2022 Feb;23(2):72. doi: 10.1038/s41576-021-00443-8. Nat Rev Genet. 2022. PMID: 34880423 No abstract available.
-
A toolkit for microbial community editing.Nat Rev Microbiol. 2022 Jul;20(7):383. doi: 10.1038/s41579-022-00747-4. Epub 2022 May 17. Nat Rev Microbiol. 2022. PMID: 35581476 No abstract available.
References
-
- Fux CA, Shirtliff M, Stoodley P & Costerton JW Can laboratory reference strains mirror ‘real-world’ pathogenesis? Trends Microbiol. 13, 58–63 (2005). - PubMed
-
- Pukall R, Tschäpe H & Smalla K Monitoring the spread of broad host and narrow host range plasmids in soil microcosms. FEMS Microbiol. Ecol. 20, 53–66 (1996).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

