Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy
- PMID: 34873307
- PMCID: PMC7612108
- DOI: 10.1038/s41551-021-00826-6
Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy
Abstract
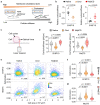
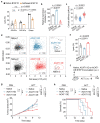
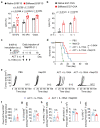
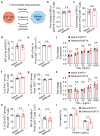
Malignant transformation and tumour progression are associated with cancer-cell softening. Yet how the biomechanics of cancer cells affects T-cell-mediated cytotoxicity and thus the outcomes of adoptive T-cell immunotherapies is unknown. Here we show that T-cell-mediated cancer-cell killing is hampered for cortically soft cancer cells, which have plasma membranes enriched in cholesterol, and that cancer-cell stiffening via cholesterol depletion augments T-cell cytotoxicity and enhances the efficacy of adoptive T-cell therapy against solid tumours in mice. We also show that the enhanced cytotoxicity against stiffened cancer cells is mediated by augmented T-cell forces arising from an increased accumulation of filamentous actin at the immunological synapse, and that cancer-cell stiffening has negligible influence on: T-cell-receptor signalling, production of cytolytic proteins such as granzyme B, secretion of interferon gamma and tumour necrosis factor alpha, and Fas-receptor-Fas-ligand interactions. Our findings reveal a mechanical immune checkpoint that could be targeted therapeutically to improve the effectiveness of cancer immunotherapies.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wang H, Mooney DJ. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat Mater. 2018;17:761–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

