The Response of Corneal Endothelial Cells to Shear Stress in an In Vitro Flow Model
- PMID: 34873452
- PMCID: PMC8643247
- DOI: 10.1155/2021/9217866
The Response of Corneal Endothelial Cells to Shear Stress in an In Vitro Flow Model
Abstract
Purpose: Corneal endothelial cells are usually exposed to shear stress caused by the aqueous humour, which is similar to the exposure of vascular endothelial cells to shear stress caused by blood flow. However, the effect of fluid shear stress on corneal endothelial cells is still poorly understood. The purpose of this study was to explore whether the shear stress that results from the aqueous humour influences corneal endothelial cells.
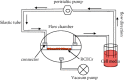
Methods: An in vitro model was established to generate fluid flow on cells, and the effect of fluid flow on corneal endothelial cells after exposure to two levels of shear stress for different durations was investigated. The mRNA and protein expression of corneal endothelium-related markers in rabbit corneal endothelial cells was evaluated by real-time PCR and western blotting.
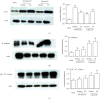
Results: The expression of the corneal endothelium-related markers ZO-1, N-cadherin, and Na+-K+-ATPase in rabbit corneal endothelial cells (RCECs) was upregulated at both the mRNA and protein levels after exposure to shear stress.
Conclusion: This study demonstrates that RCECs respond favourably to fluid shear stress, which may contribute to the maintenance of corneal endothelial cell function. Furthermore, this study also provides a theoretical foundation for further investigating the response of human corneal endothelial cells to the shear stress caused by the aqueous humour.
Copyright © 2021 Sujuan Duan et al.
Conflict of interest statement
There are no conflicts of interest.
Figures
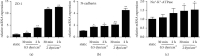
Similar articles
-
Corneal endothelial expansion using human umbilical cord mesenchymal stem cell-derived conditioned medium.J Cell Physiol. 2021 Apr;236(4):2606-2615. doi: 10.1002/jcp.30014. Epub 2020 Aug 27. J Cell Physiol. 2021. PMID: 32853402
-
Effect of anterior chamber depth on shear stress exerted on corneal endothelial cells by altered aqueous flow after laser iridotomy.Invest Ophthalmol Vis Sci. 2010 Apr;51(4):1956-64. doi: 10.1167/iovs.09-4280. Epub 2009 Nov 11. Invest Ophthalmol Vis Sci. 2010. PMID: 19907022
-
Spontaneous acquisition of infinite proliferative capacity by a rabbit corneal endothelial cell line with maintenance of phenotypic and physiological characteristics.J Tissue Eng Regen Med. 2017 Apr;11(4):1057-1064. doi: 10.1002/term.2005. Epub 2015 Mar 11. J Tissue Eng Regen Med. 2017. PMID: 25758102
-
Low shear stress up-regulation of proinflammatory gene expression in human retinal microvascular endothelial cells.Exp Eye Res. 2013 Nov;116:308-11. doi: 10.1016/j.exer.2013.10.001. Epub 2013 Oct 12. Exp Eye Res. 2013. PMID: 24128656 Review.
-
Hormonal regulation of Na+/K+-dependent ATPase activity and pump function in corneal endothelial cells.Cornea. 2011 Oct;30 Suppl 1:S60-6. doi: 10.1097/ICO.0b013e318227faab. Cornea. 2011. PMID: 21912233 Review.
Cited by
-
Unraveling the mechanobiology of cornea: From bench side to the clinic.Front Bioeng Biotechnol. 2022 Oct 3;10:953590. doi: 10.3389/fbioe.2022.953590. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36263359 Free PMC article. Review.
-
Advancements in bioengineering for descemet membrane endothelial keratoplasty (DMEK).NPJ Regen Med. 2025 Feb 14;10(1):10. doi: 10.1038/s41536-025-00396-0. NPJ Regen Med. 2025. PMID: 39952985 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

