Disentangling post-vaccination symptoms from early COVID-19
- PMID: 34873584
- PMCID: PMC8635464
- DOI: 10.1016/j.eclinm.2021.101212
Disentangling post-vaccination symptoms from early COVID-19
Abstract
Background: Identifying and testing individuals likely to have SARS-CoV-2 is critical for infection control, including post-vaccination. Vaccination is a major public health strategy to reduce SARS-CoV-2 infection globally. Some individuals experience systemic symptoms post-vaccination, which overlap with COVID-19 symptoms. This study compared early post-vaccination symptoms in individuals who subsequently tested positive or negative for SARS-CoV-2, using data from the COVID Symptom Study (CSS) app.
Methods: We conducted a prospective observational study in 1,072,313 UK CSS participants who were asymptomatic when vaccinated with Pfizer-BioNTech mRNA vaccine (BNT162b2) or Oxford-AstraZeneca adenovirus-vectored vaccine (ChAdOx1 nCoV-19) between 8 December 2020 and 17 May 2021, who subsequently reported symptoms within seven days (N=362,770) (other than local symptoms at injection site) and were tested for SARS-CoV-2 (N=14,842), aiming to differentiate vaccination side-effects per se from superimposed SARS-CoV-2 infection. The post-vaccination symptoms and SARS-CoV-2 test results were contemporaneously logged by participants. Demographic and clinical information (including comorbidities) were recorded. Symptom profiles in individuals testing positive were compared with a 1:1 matched population testing negative, including using machine learning and multiple models considering UK testing criteria.
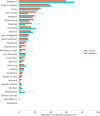
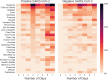
Findings: Differentiating post-vaccination side-effects alone from early COVID-19 was challenging, with a sensitivity in identification of individuals testing positive of 0.6 at best. Most of these individuals did not have fever, persistent cough, or anosmia/dysosmia, requisite symptoms for accessing UK testing; and many only had systemic symptoms commonly seen post-vaccination in individuals negative for SARS-CoV-2 (headache, myalgia, and fatigue).
Interpretation: Post-vaccination symptoms per se cannot be differentiated from COVID-19 with clinical robustness, either using symptom profiles or machine-derived models. Individuals presenting with systemic symptoms post-vaccination should be tested for SARS-CoV-2 or quarantining, to prevent community spread.
Funding: UK Government Department of Health and Social Care, Wellcome Trust, UK Engineering and Physical Sciences Research Council, UK National Institute for Health Research, UK Medical Research Council and British Heart Foundation, Chronic Disease Research Foundation, Zoe Limited.
Keywords: AUC, Area under the curve; BMI, Body mass index; CI, Confidence interval; COVID-19 detection; COVID-19, Coronavirus disease 2019; CSS, COVID Symptoms Study; DI, Data invalid; Early detection; IQR, inter quartile range; KCL, King's College London; LFAT, Lateral flow antigen test; LR, Logistic Regression; Mobile technology; NHS UK, National Health Service of the United Kingdom; O-AZ, Oxford-AstraZeneca adenovirus-vectored vaccine; PB, Pfizer-BoiNTech mRNA vaccine; RF, Random forest; ROC, Receiver operating curve; SARS-CoV-2, Severe acute respiratory syndrome-related coronavirus-2; Self-reported symptoms; Side-effects; UK, United Kingdom of Great Britain and Nothern Ireland; Vaccination; bMEM, Bayesian mixed-effect model; rtPCR, Reverse transcription polymerase chain reaction; severe acute respiratory syndrome‐related coronavirus 2 (SARS-CoV-2).
© 2021 The Authors.
Conflict of interest statement
CJS report grants from the Chronic Disease Research Foundation (CDRF), Medical Research Council (MRC) and Wellcome Trust during the conduct of the study. ELD reports being a co-lead of the KCL COVID Symptom Study Biobank, a research-supported biobank of individuals with Long COVID, administered through King's College London, during this study.EM reports grants from the MRC, as a personal fellowship. CHS reports grant from Alzheimer's Society. CH, SS, LP, AM report other from ZOE Limited and are employed by ZOE Limited, during the conduct of the study. TS is a scientific advisor to ZOE Limited. CHS reports grants from Alzheimer's Society, during the conduct of the study. SO reports grants from the Wellcome Trust, Innovate UK (UKRI), and Chronic Disease Research Foundation (CDRF), during the conduct of the study. All the other authors have no conflicts.
Figures


References
-
- Worldometer COVID-19 Coronavirus Pandemic. Oct 13, 2020 https://www.worldometers.info/coronavirus/ accessed.
-
- World Health Organisation WHO Coronavirus (COVID-19) Dashboard. 2021 published online May 25.
-
- Medicines and Healthcare products Regulatory Agency Public Assessment Report Authorisation for Temporary Supply COVID-19 mRNA Vaccine BNT162b2 (BNT162b2 RNA) concentrate for solution for injection. May 26, 2021 https://assets.publishing.service.gov.uk/government/uploads/system/uploa... accessed.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

