Pharmacology Versus Convenience: A Benefit/Risk Analysis of Regular Maintenance Versus Infrequent or As-Needed Inhaled Corticosteroid Use in Mild Asthma
- PMID: 34873657
- PMCID: PMC8799535
- DOI: 10.1007/s12325-021-01976-4
Pharmacology Versus Convenience: A Benefit/Risk Analysis of Regular Maintenance Versus Infrequent or As-Needed Inhaled Corticosteroid Use in Mild Asthma
Abstract
Introduction: This study compared the bronchoprotective and benefit/risk profiles of various inhaled corticosteroid (ICS) dosing regimens in mild asthma.
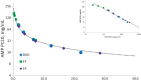
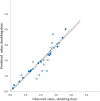
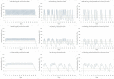
Methods: A pharmacokinetic/pharmacodynamic model was developed and validated describing the relationship between ICS dose and time-course for airway bronchoprotection, [provocative concentration of adenosine monophosphate (AMP) causing ≥ 20% decline in forced expiratory volume in 1 s (FEV1) (AMP PC20)], for fluticasone furoate (FF), fluticasone propionate (FP) and budesonide (BUD). For regular ICS maintenance therapy (100% and 50% adherence) and infrequent or as-needed use (dosing 3-4 times per week), treatment effectiveness was expressed as percent time during 28 days when bronchoprotection exceeded either the threshold for a treatment-related bronchoprotective effect (AMP PC20 ≥ 0.25 doubling dose) or the threshold for a clinically significant bronchoprotective effect (AMP PC20 ≥ 1.0 doubling dose). This value was divided by the total ICS dose administered expressed in prednisolone equivalents to give a therapeutic index (TI).
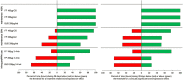
Results: The model-predicted time course of ICS-induced bronchoprotection with regular daily maintenance dosing and 100% adherence showed that all ICS at the highest recommended doses for mild asthma exceeded the threshold for clinically significant bronchoprotective effect for all or most of the 28-day dosing period, mean (90% CI); 100% (96.1-100), 99.9% (8.0-100) and 100% (58.2-100) with TI values of 16.9, 6.6 and 5.4 for FF 100 µg OD, FP 200 µg BID and BUD 200 µg BID, respectively. For simulated poor adherence (50%) to regular daily maintenance therapy, corresponding mean (90% CI) values were; 75.7% (39.4-89.1), 52.3% (0.7-69.2) and 51.3% (28.6-58.3) with TI values of 25.7, 6.9 and 5.6. For simulated infrequent/as needed use the corresponding values were; 77.0% (37.6-87.0), 25.5% (0.0-38.0) and 26.2% (14.3-31.5) with TI values of 26.1, 6.7 and 5.7. For all regimen/scenarios, FF had the most sustained efficacy and favourable TI followed by FP and BUD.
Conclusions: At doses recommended for mild asthma, all ICS regimens provide sustained bronchoprotective efficacy when dosed regularly with high adherence. With poor adherence or use 3-4 times per week (infrequent/as needed), longer-acting ICS molecules will more likely provide sustained protection and a better TI versus shorter duration of action molecules (FF > FP ≥ BUD). These data highlight the benefits of using ICS as regular daily maintenance dosing in mild asthma and the potential risks of under-treatment with ICS (which may occur with ICS/formoterol as-needed approach in mild persistent asthma) associated with reduced levels of bronchoprotection.
Keywords: AMP challenge; Adherence; As-needed; Asthma; Budesonide; Fluticasone furoate; Fluticasone propionate; PK/PD model; Regular dosing.
© 2021. The Author(s).
Figures

Similar articles
-
New Versus Old: The Impact of Changing Patterns of Inhaled Corticosteroid Prescribing and Dosing Regimens in Asthma Management.Adv Ther. 2022 May;39(5):1895-1914. doi: 10.1007/s12325-022-02092-7. Epub 2022 Mar 14. Adv Ther. 2022. PMID: 35284999 Free PMC article. Review.
-
Assessing the Effects of Changing Patterns of Inhaled Corticosteroid Dosing and Adherence with Fluticasone Furoate and Budesonide on Asthma Management.Adv Ther. 2023 Sep;40(9):4042-4059. doi: 10.1007/s12325-023-02585-z. Epub 2023 Jul 12. Adv Ther. 2023. PMID: 37438554 Free PMC article. Review.
-
Therapeutic index of inhaled corticosteroids in asthma: A dose-response comparison on airway hyperresponsiveness and adrenal axis suppression.Br J Clin Pharmacol. 2021 Feb;87(2):483-493. doi: 10.1111/bcp.14406. Epub 2020 Jun 17. Br J Clin Pharmacol. 2021. PMID: 32484940 Free PMC article. Clinical Trial.
-
The CONCEPT trial: a 1-year, multicenter, randomized,double-blind, double-dummy comparison of a stable dosing regimen of salmeterol/fluticasone propionate with an adjustable maintenance dosing regimen of formoterol/budesonide in adults with persistent asthma.Clin Ther. 2005 Apr;27(4):393-406. doi: 10.1016/j.clinthera.2005.03.006. Clin Ther. 2005. PMID: 15922813 Clinical Trial.
-
Budesonide/formoterol maintenance and reliever therapy in Asian patients (aged ≥16 years) with asthma: a sub-analysis of the COSMOS study.Clin Drug Investig. 2012 Jul 1;32(7):439-49. doi: 10.2165/11598840-000000000-00000. Clin Drug Investig. 2012. PMID: 22607479 Clinical Trial.
Cited by
-
Are Treatment Adherence Factors Apparent in Patients with Asthma and to Physicians? Results from the APPaRENT 3 Survey.Adv Ther. 2025 Mar;42(3):1506-1521. doi: 10.1007/s12325-025-03105-x. Epub 2025 Feb 6. Adv Ther. 2025. PMID: 39912987 Free PMC article.
-
Inhaled Corticosteroids in Subjects with Chronic Obstructive Pulmonary Disease: An Old, Unfinished History.Biomolecules. 2024 Feb 6;14(2):195. doi: 10.3390/biom14020195. Biomolecules. 2024. PMID: 38397432 Free PMC article. Review.
-
Budesonide Attains Its Wide Clinical Profile by Alternative Kinetics.Pharmaceuticals (Basel). 2024 Apr 15;17(4):503. doi: 10.3390/ph17040503. Pharmaceuticals (Basel). 2024. PMID: 38675463 Free PMC article. Review.
-
The Changing Asthma Management Landscape and Need for Appropriate SABA Prescription.Adv Ther. 2023 Apr;40(4):1301-1316. doi: 10.1007/s12325-022-02410-z. Epub 2023 Jan 30. Adv Ther. 2023. PMID: 36715896 Free PMC article.
-
Expert opinion on gray areas in asthma management: A lesson from the innovative project "revolution in asthma" of the Italian thoracic society (AIPO-ITS).Clin Transl Allergy. 2025 Feb;15(2):e70037. doi: 10.1002/clt2.70037. Clin Transl Allergy. 2025. PMID: 39924642 Free PMC article. Review.
References
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention. Global Initiative for Asthma (GINA), 2021. https://ginasthma.org/gina-reports/.
-
- The British Thoracic Society. BTS/SIGN British guideline on the management of asthma, 2019. https://www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-br....
-
- Busse WW, Pedersen S, Pauwels RA, START Investigators Group et al. The inhaled steroid treatment as regular therapy in early asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol. 2008;121(5):1167–1174. - PubMed
-
- O'Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW, START Investigators Group Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19–24. - PubMed
-
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

