Brain opioid segments and striatal patterns of dopamine release induced by naloxone and morphine
- PMID: 34873784
- PMCID: PMC8837588
- DOI: 10.1002/hbm.25733
Brain opioid segments and striatal patterns of dopamine release induced by naloxone and morphine
Abstract
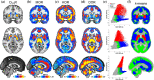
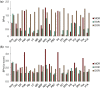
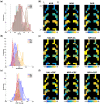
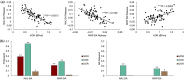
Opioid receptors are expressed throughout the brain and play a major role in regulating striatal dopamine (DA) release. Clinical studies have shown that naloxone (NAL, a nonspecific opioid antagonist) in individuals with opioid use disorder and morphine (MRP, a nonspecific opioid agonist) in healthy controls, resulted in DA release in the dorsal and ventral striatum, respectively. It is not known whether the underlying patterns of striatal DA release are associated with the striatal distribution of opioid receptors. We leveraged previously published PET datasets (collected in independent cohorts) to study the brain-wide distribution of opioid receptors and to compare striatal opioid receptor availability with striatal DA release patterns. We identified three major gray matter segments based on availability maps of DA and opioid receptors: striatum, and primary and secondary opioid segments with high and intermediate opioid receptor availability, respectively. Patterns of DA release induced by NAL and MRP were inversely associated and correlated with kappa (NAL: r(68) = -0.81, MRP: r(68) = 0.54), and mu (NAL: r(68) = -0.62, MRP: r(68) = 0.46) opioid receptor availability. Kappa opioid receptor availability accounted for a unique part of variance in NAL- and MRP-DA release patterns (ΔR2 >0.14, p <.0001). In sum, distributions of opioid receptors distinguished major cortical and subcortical regions. Patterns of NAL- and MRP-induced DA release had inverse associations with striatal opioid receptor availability. Our approach provides a pattern-based characterization of drug-induced DA targets and is relevant for modeling the role of opioid receptors in modulating striatal DA release.
Keywords: dopamine; k-means; morphine; naloxone; opioid receptors; positron emission tomography (PET); striatum.
© 2021 The Authors. Human Brain Mapping published by Wiley Periodicals LLC. This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
References
-
- Bilgel, M. , Beason‐Held, L. , An, Y. , Zhou, Y. , Wong, D. F. , & Resnick, S. M. (2020). Longitudinal evaluation of surrogates of regional cerebral blood flow computed from dynamic amyloid PET imaging. Journal of Cerebral Blood Flow and Metabolism, 40(2), 288–297. 10.1177/0271678x19830537 - DOI - PMC - PubMed
-
- Birn, R. M. , Converse, A. K. , Rajala, A. Z. , Alexander, A. L. , Block, W. F. , McMillan, A. B. , … Populin, L. C. (2019). Changes in endogenous dopamine induced by methylphenidate predict functional connectivity in nonhuman primates. The Journal of Neuroscience, 39(8), 1436–1444. 10.1523/jneurosci.2513-18.2018 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

