Hepatitis D virus (HDV) prevalence in Austria is low but causes considerable morbidity due to fast progression to cirrhosis
- PMID: 34873866
- PMCID: PMC8672089
- DOI: 10.1002/ueg2.12163
Hepatitis D virus (HDV) prevalence in Austria is low but causes considerable morbidity due to fast progression to cirrhosis
Abstract
Background: Hepatitis D virus (HDV) coinfection aggravates the course of hepatitis B virus (HBV). The prevalence of HDV in Austria is unknown.
Objective: This national study aimed at (i) recording the prevalence of HDV-infection in Austria and (ii) characterizing the "active" HDV cohort in Austria.
Methods: A total of 10 hepatitis treatment centers in Austria participated in this multicenter study and retrospectively collected their HDV patients between Q1/2010 and Q4/2020. Positive anti-HDV and/or HDV-RNA-polymerase chain reaction (PCR) results were retrieved from local database queries. Disease severity was assessed by individual chart review. Viremic HDV patients with clinical visits in/after Q1/2019 were considered as the "active" HDV cohort.
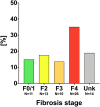
Results: A total of 347 anti-HDV positive patients were identified. In 202 (58.2%) patients, HDV-RNA-PCR test was performed, and 126/202 (62.4%) had confirmed viremia. Hepatocellular carcinoma was diagnosed in 7 (5.6%) patients, 7 (5.6%) patients underwent liver transplantation, and 11 (8.7%) patients died during follow-up. The "active" Austrian HDV cohort included 74 (58.7%) patients: Evidence for advanced chronic liver disease (ACLD, i.e., histological F3/F4 fibrosis, liver stiffness ≥10 kPa, varices, or hepatic venous pressure gradient ≥6 mmHg) was detected in 38 (51.4%) patients, including 2 (5.3%) with decompensation (ascites/hepatic encephalopathy). About 37 (50.0%) patients of the "active" HDV cohort had previously received interferon treatment. Treatment with the sodium-taurocholate cotransporting polypeptide inhibitor bulevirtide was initiated in 20 (27.0%) patients.
Conclusion: The number of confirmed HDV viremic cases in Austria is low (<1% of HBV patients) but potentially underestimated. Testing all HBV patients will increase the diagnostic yield. More than half of viremic HDV patients had ACLD. Improved HDV testing and workup strategies will facilitate access to novel antiviral therapies.
Keywords: epidemiology; hepatitis D; viral hepatitis.
© 2021 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
Caroline Schmidbauer received travel support from Gilead, Abbvie and Gebro; and speaking honoraria from Abbvie. Stephanie Hametner‐Schreil received speaking honoraria and/or advisory board fees from: Gilead, Abbvie, Shionogi, Astellas, Intercept, Roche. Petra Munda received speaking honoraria from AbbVie, Gilead, MSD, Janssen, Roche, Intercept; travel support from AbbVie and Gilead; and consulting/advisory board fees from AbbVie, Gilead, MSD, Janssen, Roche and Intercept. M.M. served as a speaker and/or consultant and/or advisory board member for AbbVie, Bristol‐Myers Squibb, Gilead, Collective Acumen, and W. L. Gore & Associates and received travel support from AbbVie, Bristol‐Myers Squibb, and Gilead. M.P.‐R. received advisory and/or speaker honoraria from AstraZeneca, AbbVie, Amgen, Bayer, Behring, BMS, Eisai, Gilead, Intercept, Ipsen, Lilly, Merz, MSD, Roche, Sanofi, Shionogi, Sobi. M.G. received grants from AbbVie, Gilead, and MSD; speaking honoraria from AbbVie, Gilead, MSD, Janssen, Roche, Intercept; and consulting/advisory board fees from AbbVie, Gilead, MSD, Janssen, Roche and intercept. Peter Ferenci received an unrestricted research grant from Gilead, is on the safety review board of MyrPharma, consulting/advisory board fees from Viravaxx, speaking honoraria from AbbVie, Gilead. T.R. served as a speaker and/or consultant and/or advisory board member for AbbVie, Bayer, Boehringer Ingelheim, Gilead, Intercept, MSD, Siemens, and W. L. Gore & Associates and received grants/research support from AbbVie, Boehringer Ingelheim, Gilead, MSD, Philips, and W.L. Gore & Associates as well as travel support from Boehringer Ingelheim and Gilead. Mathias Jachs, Teresa Binter, Lukas Hartl, Michael Strasser, Hermann Laferl, Alexander Lindorfer, Kristina Dax, Rudolf E. Stauber, Harald H. Kessler, Sebastian Bernhofer, Andreas Maieron, Lorin Loacker, Simona Bota, Isabel Santonja, and Heidemarie Holzmann declare no conflicts of interest.
Figures



Comment in
-
Prevalence of hepatitis D virus co-infection in Austria - Finding the needle in the haystack.United European Gastroenterol J. 2021 Dec;9(10):1105-1106. doi: 10.1002/ueg2.12177. Epub 2021 Nov 24. United European Gastroenterol J. 2021. PMID: 34817935 Free PMC article. No abstract available.
References
-
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55. - PubMed
-
- Gheorghe L, Csiki IE, Iacob S, Gheorghe C, Trifan A, Grigorescu M, et al. Hepatitis delta virus infection in Romania: prevalence and Risk Factors. J Gastrointestin Liver Dis. 2015;24(4):413–21. - PubMed
-
- Yesmembetov KI, Mukhin NA, Abdurakhmanov DT. 160 hepatitis delta—still of major concern in Russia, due to immigration and intravenous drug usage: view from large single center cohort. J Hepatol. 2012;56:69.
-
- Khodjaeva M, Ibadullaeva N, Khikmatullaeva A, Joldasova E, Ismoilov U, Colombo M, et al. The medical impact of hepatitis D virus infection in Uzbekistan. Liver Int. 2019;39(11):2077–81. - PubMed
-
- Wranke A, Pinheiro Borzacov LM, Parana R, Lobato C, Hamid S, Ceausu E, et al. Clinical and virological heterogeneity of hepatitis delta in different regions world‐wide: the Hepatitis Delta International Network (HDIN). Liver Int. 2018;38(5):842–50. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

