Challenges for assessing replicability in preclinical cancer biology
- PMID: 34874008
- PMCID: PMC8651289
- DOI: 10.7554/eLife.67995
Challenges for assessing replicability in preclinical cancer biology
Abstract
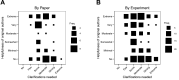
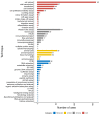
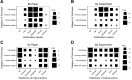
We conducted the Reproducibility Project: Cancer Biology to investigate the replicability of preclinical research in cancer biology. The initial aim of the project was to repeat 193 experiments from 53 high-impact papers, using an approach in which the experimental protocols and plans for data analysis had to be peer reviewed and accepted for publication before experimental work could begin. However, the various barriers and challenges we encountered while designing and conducting the experiments meant that we were only able to repeat 50 experiments from 23 papers. Here we report these barriers and challenges. First, many original papers failed to report key descriptive and inferential statistics: the data needed to compute effect sizes and conduct power analyses was publicly accessible for just 4 of 193 experiments. Moreover, despite contacting the authors of the original papers, we were unable to obtain these data for 68% of the experiments. Second, none of the 193 experiments were described in sufficient detail in the original paper to enable us to design protocols to repeat the experiments, so we had to seek clarifications from the original authors. While authors were extremely or very helpful for 41% of experiments, they were minimally helpful for 9% of experiments, and not at all helpful (or did not respond to us) for 32% of experiments. Third, once experimental work started, 67% of the peer-reviewed protocols required modifications to complete the research and just 41% of those modifications could be implemented. Cumulatively, these three factors limited the number of experiments that could be repeated. This experience draws attention to a basic and fundamental concern about replication - it is hard to assess whether reported findings are credible.
Keywords: Reproducibility Project: Cancer Biology; cancer biology; human; mouse; open data; open science; preregistration; replication; reproducibility.
© 2021, Errington et al.
Conflict of interest statement
TE, AD, BN Employed by the nonprofit Center for Open Science that has a mission to increase openness, integrity, and reproducibility of research, NP, EI Employed by and hold shares in Science Exchange Inc
Figures


Comment in
-
Half of top cancer studies fail high-profile reproducibility effort.Nature. 2021 Dec;600(7889):368-369. doi: 10.1038/d41586-021-03691-0. Nature. 2021. PMID: 34887584 No abstract available.
References
-
- Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

