Electrochemical and spectroelectrochemical characterization of bacteria and bacterial systems
- PMID: 34874024
- PMCID: PMC8791413
- DOI: 10.1039/d1an01954f
Electrochemical and spectroelectrochemical characterization of bacteria and bacterial systems
Abstract
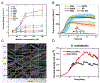
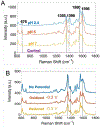
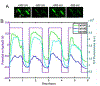
Microbes, such as bacteria, can be described, at one level, as small, self-sustaining chemical factories. Based on the species, strain, and even the environment, bacteria can be useful, neutral or pathogenic to human life, so it is increasingly important that we be able to characterize them at the molecular level with chemical specificity and spatial and temporal resolution in order to understand their behavior. Bacterial metabolism involves a large number of internal and external electron transfer processes, so it is logical that electrochemical techniques have been employed to investigate these bacterial metabolites. In this mini-review, we focus on electrochemical and spectroelectrochemical methods that have been developed and used specifically to chemically characterize bacteria and their behavior. First, we discuss the latest mechanistic insights and current understanding of microbial electron transfer, including both direct and mediated electron transfer. Second, we summarize progress on approaches to spatiotemporal characterization of secreted factors, including both metabolites and signaling molecules, which can be used to discern how natural or external factors can alter metabolic states of bacterial cells and change either their individual or collective behavior. Finally, we address in situ methods of single-cell characterization, which can uncover how heterogeneity in cell behavior is reflected in the behavior and properties of collections of bacteria, e.g. bacterial communities. Recent advances in (spectro)electrochemical characterization of bacteria have yielded important new insights both at the ensemble and the single-entity levels, which are furthering our understanding of bacterial behavior. These insights, in turn, promise to benefit applications ranging from biosensors to the use of bacteria in bacteria-based bioenergy generation and storage.
Conflict of interest statement
Conflicts of interest
The authors declare no conflict of interest.
Figures
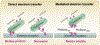



Similar articles
-
Electrochemical Probes of Microbial Community Behavior.Annu Rev Anal Chem (Palo Alto Calif). 2018 Jun 12;11(1):441-461. doi: 10.1146/annurev-anchem-061417-125627. Epub 2018 Feb 28. Annu Rev Anal Chem (Palo Alto Calif). 2018. PMID: 29490192 Review.
-
Recent Advances in CMOS Electrochemical Biosensor Design for Microbial Monitoring: Review and Design Methodology.IEEE Trans Biomed Circuits Syst. 2023 Apr;17(2):202-228. doi: 10.1109/TBCAS.2023.3252402. Epub 2023 May 10. IEEE Trans Biomed Circuits Syst. 2023. PMID: 37028090 Review.
-
High-Spatiotemporal-Resolution Electrochemical Measurements of Electrocatalytic Reactivity.Anal Chem. 2023 Apr 25;95(16):6477-6489. doi: 10.1021/acs.analchem.2c05755. Epub 2023 Apr 6. Anal Chem. 2023. PMID: 37023363 Review.
-
Reconstitution of supramolecular organization involved in energy metabolism at electrochemical interfaces for biosensing and bioenergy production.Anal Bioanal Chem. 2014 Feb;406(4):1011-27. doi: 10.1007/s00216-013-7465-1. Epub 2013 Dec 1. Anal Bioanal Chem. 2014. PMID: 24292430 Review.
-
Investigating Nanoscale Electrochemistry with Surface- and Tip-Enhanced Raman Spectroscopy.Acc Chem Res. 2016 Sep 20;49(9):2023-30. doi: 10.1021/acs.accounts.6b00327. Epub 2016 Sep 7. Acc Chem Res. 2016. PMID: 27602428
Cited by
-
Computer-Assisted Processing of Current Step Signals in Single Blocking Impact Electrochemistry.ACS Meas Sci Au. 2024 Sep 6;4(5):585-592. doi: 10.1021/acsmeasuresciau.4c00046. eCollection 2024 Oct 16. ACS Meas Sci Au. 2024. PMID: 39430961 Free PMC article.
-
Trends in single-impact electrochemistry for bacteria analysis.Anal Bioanal Chem. 2023 Jul;415(18):3717-3725. doi: 10.1007/s00216-023-04568-z. Epub 2023 Feb 9. Anal Bioanal Chem. 2023. PMID: 36754873 Review.
-
Enhanced spectroelectrochemistry with lossy-mode resonance optical fiber sensor.Sci Rep. 2023 Sep 19;13(1):15523. doi: 10.1038/s41598-023-42853-0. Sci Rep. 2023. PMID: 37726408 Free PMC article.
-
Plasma Functionalized Carbon Interfaces for Biosensor Application: Toward the Real-Time Detection of Escherichia coli O157:H7.ACS Omega. 2022 Jun 6;7(24):21025-21034. doi: 10.1021/acsomega.2c01802. eCollection 2022 Jun 21. ACS Omega. 2022. PMID: 35755381 Free PMC article.
References
-
- Lorenzo JM, Munekata PE, Dominguez R, Pateiro M, Saraiva JA and Franco D, in Innovative Technologies for Food Preservation, eds. Barba FJ, Sant’Ana AS, Orlien V and Koubaa M, Academic Press, 2018, DOI: 10.1016/B978-0-12-811031-7.00003-0, pp. 53–107. - DOI
-
- Gram L, Ravn L, Rasch M, Bruhn JB, Christensen AB and Givskov M, International Journal of Food Microbiology, 2002, 78, 79–97. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

