Racial and Ethnic Representation in US Clinical Trials of New Drugs and Biologics, 2015-2019
- PMID: 34874429
- PMCID: PMC8652601
- DOI: 10.1001/jama.2021.16680
Racial and Ethnic Representation in US Clinical Trials of New Drugs and Biologics, 2015-2019
Abstract
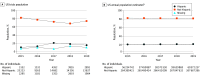
This study reviews the participation of racial and ethnic populations at US sites in 2015-2019 to understand the extent to which US trial participation represents the diversity of the US population.
Conflict of interest statement
Figures

Comment in
-
Racial and Ethnic Representation of Participants in US Clinical Trials of New Drugs and Biologics.JAMA. 2022 Mar 8;327(10):985. doi: 10.1001/jama.2022.0875. JAMA. 2022. PMID: 35258537 No abstract available.
References
-
- US Food and Drug Administration . 2015-2019 Drug trials snapshots: summary report. Accessed March 9, 2021. https://www.fda.gov/media/143592/download
-
- US Food and Drug Administration . Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. Published October 2016. Accessed March 10, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
-
- US Census Bureau . National population by characteristics: 2010-2019. Accessed July 2, 2021. https://www.census.gov/data/datasets/time-series/demo/popest/2010s-natio...
-
- Clinical trials have far too little racial and ethnic diversity. Scientific American. Published September 1, 2018. Accessed March 9, 2021. https://www.scientificamerican.com/article/clinical-trials-have-far-too-...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

