Imaging of peripheral vascular malformations - current concepts and future perspectives
- PMID: 34874510
- PMCID: PMC8651875
- DOI: 10.1186/s40348-021-00132-w
Imaging of peripheral vascular malformations - current concepts and future perspectives
Abstract
Vascular Malformations belong to the spectrum of orphan diseases and can involve all segments of the vascular tree: arteries, capillaries, and veins, and similarly the lymphatic vasculature. The classification according to the International Society for the Study of Vascular Anomalies (ISSVA) is of major importance to guide proper treatment. Imaging plays a crucial role to classify vascular malformations according to their dominant vessel type, anatomical extension, and flow pattern. Several imaging concepts including color-coded Duplex ultrasound/contrast-enhanced ultrasound (CDUS/CEUS), 4D computed tomography angiography (CTA), magnetic resonance imaging (MRI) including dynamic contrast-enhanced MR-angiography (DCE-MRA), and conventional arterial and venous angiography are established in the current clinical routine. Besides the very heterogenous phenotypes of vascular malformations, molecular and genetic profiling has recently offered an advanced understanding of the pathogenesis and progression of these lesions. As distinct molecular subtypes may be suitable for targeted therapies, capturing certain patterns by means of molecular imaging could enhance non-invasive diagnostics of vascular malformations. This review provides an overview of subtype-specific imaging and established imaging modalities, as well as future perspectives of novel functional and molecular imaging approaches. We highlight recent pioneering imaging studies including thermography, positron emission tomography (PET), and multispectral optoacoustic tomography (MSOT), which have successfully targeted specific biomarkers of vascular malformations.
Keywords: Duplex ultrasound; Magnetic resonance imaging; Molecular imaging; Multispectral optoacoustic tomography; Positron emission tomography; Thermography; Vascular malformations.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


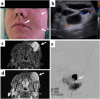
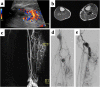

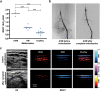
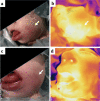
References
-
- ISSVA Classification of Vascular Anomalies @2018 International Society for the Study of Vascular Anomalies. Available at: issva.org/classification. Accessed 16 April 2021
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

