Uveitis-mediated immune cell invasion through the extracellular matrix of the lens capsule
- PMID: 34874579
- PMCID: PMC9300120
- DOI: 10.1096/fj.202101098R
Uveitis-mediated immune cell invasion through the extracellular matrix of the lens capsule
Abstract
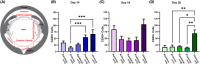
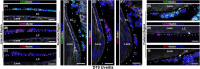
While the eye is considered an immune privileged site, its privilege is abrogated when immune cells are recruited from the surrounding vasculature in response to trauma, infection, aging, and autoimmune diseases like uveitis. Here, we investigate whether in uveitis immune cells become associated with the lens capsule and compromise its privilege in studies of C57BL/6J mice with experimental autoimmune uveitis. These studies show that at D14, the peak of uveitis in these mice, T cells, macrophages, and Ly6G/Ly6C+ immune cells associate with the lens basement membrane capsule, burrow into the capsule matrix, and remain integrated with the capsule as immune resolution is occurring at D26. 3D surface rendering image analytics of confocal z-stacks and scanning electron microscopy imaging of the lens surface show the degradation of the lens capsule as these lens-associated immune cells integrate with and invade the lens capsule, with a subset infiltrating both epithelial and fiber cell regions of lens tissue, abrogating its immune privilege. Those immune cells that remain on the surface often become entwined with a fibrillar net-like structure. Immune cell invasion of the lens capsule in uveitis has not been described previously and may play a role in induction of lens and other eye pathologies associated with autoimmunity.
Keywords: basement membrane; immune cell attachment; immune cell infiltration; immune cell migration; immune privilege; inflammation; lens; matrix; uveitic.
© 2021 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures






Similar articles
-
Immunoregulatory Properties of Immune Cells that Associate with the Lens Capsule Surface during Acute and Resolution Phases of Experimental Autoimmune Uveitis.Am J Pathol. 2024 Nov;194(11):2194-2211. doi: 10.1016/j.ajpath.2024.07.021. Epub 2024 Aug 17. Am J Pathol. 2024. PMID: 39159867 Free PMC article.
-
Immunoregulatory role of ocular macrophages: the macrophages produce RANTES to suppress experimental autoimmune uveitis.J Immunol. 2003 Sep 1;171(5):2652-9. doi: 10.4049/jimmunol.171.5.2652. J Immunol. 2003. PMID: 12928419
-
[The etiopathology of phacoantigenic uveitis and phacolytic glaucoma].Oftalmologia. 1992 Jul-Sep;36(3):207-13. Oftalmologia. 1992. PMID: 1520677 Review. Romanian.
-
Spontaneous development of autoimmune uveitis Is CCR2 dependent.Am J Pathol. 2014 Jun;184(6):1695-705. doi: 10.1016/j.ajpath.2014.02.024. Epub 2014 Apr 13. Am J Pathol. 2014. PMID: 24736166 Free PMC article.
-
Lens-induced uveitis: an update.Graefes Arch Clin Exp Ophthalmol. 2020 Jul;258(7):1359-1365. doi: 10.1007/s00417-019-04598-3. Epub 2020 Jan 6. Graefes Arch Clin Exp Ophthalmol. 2020. PMID: 31907641 Free PMC article. Review.
Cited by
-
Clearing the light path: proteoglycans and their important roles in the lens and cornea.Proteoglycan Res. 2024 Apr-Jun;2(2):e20. doi: 10.1002/pgr2.20. Epub 2024 May 8. Proteoglycan Res. 2024. PMID: 39568541 Free PMC article.
-
Ex Vivo Microsuturing of the Human Anterior Lens Capsule Using 10-0 Sutures: A Technical Feasibility Report.Cureus. 2025 Apr 26;17(4):e83028. doi: 10.7759/cureus.83028. eCollection 2025 Apr. Cureus. 2025. PMID: 40432653 Free PMC article.
-
Immune Responses Induced at One Hour Post Cataract Surgery Wounding of the Chick Lens.Biomolecules. 2023 Nov 4;13(11):1615. doi: 10.3390/biom13111615. Biomolecules. 2023. PMID: 38002297 Free PMC article.
-
Associations of severe liver diseases with cataract using data from UK Biobank: a prospective cohort study.EClinicalMedicine. 2024 Jan 21;68:102424. doi: 10.1016/j.eclinm.2024.102424. eCollection 2024 Feb. EClinicalMedicine. 2024. PMID: 38304745 Free PMC article.
-
Translational regulation of cell invasion through extracellular matrix-an emerging role for ribosomes.F1000Res. 2023 Nov 29;12:1528. doi: 10.12688/f1000research.143519.1. eCollection 2023. F1000Res. 2023. PMID: 38628976 Free PMC article. Review.
References
-
- Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74:179‐185. - PubMed
-
- Rosenbaum JT, Rosenzweig HL, Smith JR, Martin TM, Planck SR. Uveitis secondary to bacterial products. Ophthalmic Res. 2008;40:165‐168. - PubMed
-
- Sharma SM, Jackson D. Uveitis and spondyloarthropathies. Best Pract Res Clin Rheumatol. 2017;31:846‐862. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

