Epidermal growth factor/epidermal growth factor receptor signaling blockage inhibits tumor cell-derived exosome uptake by oral squamous cell carcinoma through macropinocytosis
- PMID: 34874595
- PMCID: PMC8819298
- DOI: 10.1111/cas.15225
Epidermal growth factor/epidermal growth factor receptor signaling blockage inhibits tumor cell-derived exosome uptake by oral squamous cell carcinoma through macropinocytosis
Abstract
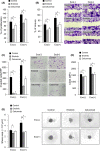
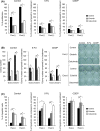
Various cell types secrete exosomes into their surrounding extracellular space, which consequently affect the function and activity of recipient cells. Numerous studies have showed that tumor cell-derived exosomes play important roles in tumor growth and progression. Although a variety of endocytic pathways are reportedly involved in the cellular uptake of exosomes, detailed mechanisms remain unknown. The present study demonstrated that treatment with recombinant epidermal growth factor (EGF) time- and dose-dependently promoted cellular uptake of oral squamous cell carcinoma (OSCC) cell-derived exosomes into OSCC cells themselves. Conversely, EGF receptor (EGFR) knockdown and treatment with EGFR inhibitors, including erlotinib and cetuximab, abrogated OSCC cell uptake of exosomes. The macropinocytosis inhibitor 5-(N-ethyl-N-isopropyl) amiloride (EIPA) blocked the effects of active EGF/EGFR signaling on uptake of OSCC cell-derived exosomes. These EGFR inhibitors also suppressed OSCC cell-derived exosome-induced proliferation, migration, invasion, stemness, and chemoresistance of OSCC cells. Taken together, the data presented herein suggest that EGFR inhibitors might inhibit the malignant potential of OSCC cells through direct inhibition of not only EGFR downstream signaling pathway but also cellular uptake of OSCC cell-derived exosomes through macropinocytosis.
Keywords: EGF; EGFR; exosome; macropinocytosis; oral squamous cell carcinoma.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures





References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Okura M, Aikawa T, Sawai NY, Iida S, Kogo M. Decision analysis and treatment threshold in a management for the N0 neck of the oral cavity carcinoma. Oral Oncol. 2009;45:908‐911. - PubMed
-
- da Silva SD, Ferlito A, Takes RP, et al. Advances and applications of oral cancer basic research. Oral Oncol. 2011;47:783‐791. - PubMed
-
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extra‐cellular organelles important in intercellular communication. J Proteomics. 2010;73:1907‐1920. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

