JAB1 deletion in oligodendrocytes causes senescence-induced inflammation and neurodegeneration in mice
- PMID: 34874913
- PMCID: PMC8803330
- DOI: 10.1172/JCI145071
JAB1 deletion in oligodendrocytes causes senescence-induced inflammation and neurodegeneration in mice
Abstract
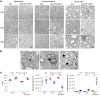
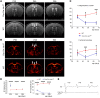
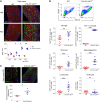
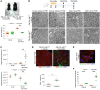
Oligodendrocytes are the primary target of demyelinating disorders, and progressive neurodegenerative changes may evolve in the CNS. DNA damage and oxidative stress are considered key pathogenic events, but the underlying molecular mechanisms remain unclear. Moreover, animal models do not fully recapitulate human diseases, complicating the path to effective treatments. Here we report that mice with cell-autonomous deletion of the nuclear COP9 signalosome component CSN5 (JAB1) in oligodendrocytes develop DNA damage and defective DNA repair in myelinating glial cells. Interestingly, oligodendrocytes lacking JAB1 expression underwent a senescence-like phenotype that fostered chronic inflammation and oxidative stress. These mutants developed progressive CNS demyelination, microglia inflammation, and neurodegeneration, with severe motor deficits and premature death. Notably, blocking microglia inflammation did not prevent neurodegeneration, whereas the deletion of p21CIP1 but not p16INK4a pathway ameliorated the disease. We suggest that senescence is key to sustaining neurodegeneration in demyelinating disorders and may be considered a potential therapeutic target.
Keywords: Cellular senescence; Demyelinating disorders; Inflammation; Mouse models; Neuroscience.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

