Astrocytic 4R tau expression drives astrocyte reactivity and dysfunction
- PMID: 34874917
- PMCID: PMC8765054
- DOI: 10.1172/jci.insight.152012
Astrocytic 4R tau expression drives astrocyte reactivity and dysfunction
Abstract
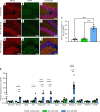
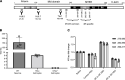
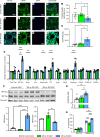
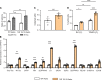
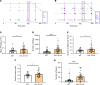
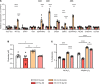
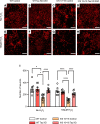
The protein tau and its isoforms are associated with several neurodegenerative diseases, many of which are characterized by greater deposition of the 4-repeat (4R) tau isoform; however, the role of 4R tau in disease pathogenesis remains unclear. We created antisense oligonucleotides (ASOs) that alter the ratio of 3R to 4R tau to investigate the role of specific tau isoforms in disease. Preferential expression of 4R tau in human tau-expressing (hTau-expressing) mice was previously shown to increase seizure severity and phosphorylated tau deposition without neuronal or synaptic loss. In this study, we observed strong colocalization of 4R tau within reactive astrocytes and increased expression of pan-reactive and neurotoxic genes following 3R to 4R tau splicing ASO treatment in hTau mice. Increasing 4R tau levels in primary astrocytes provoked a similar response, including a neurotoxic genetic profile and diminished homeostatic function, which was replicated in human induced pluripotent stem cell-derived (iPSC-derived) astrocytes harboring a mutation that exhibits greater 4R tau. Healthy neurons cultured with 4R tau-expressing human iPSC-derived astrocytes exhibited a higher firing frequency and hypersynchrony, which could be prevented by lowering tau expression. These findings support a potentially novel pathway by which astrocytic 4R tau mediates reactivity and dysfunction and suggest that astrocyte-targeted therapeutics against 4R tau may mitigate neurodegenerative disease progression.
Keywords: Neurodegeneration; Neurological disorders; Neuroscience; iPS cells.
Conflict of interest statement
Figures

Similar articles
-
Determinants of astrocytic pathology in stem cell models of primary tauopathies.Acta Neuropathol Commun. 2023 Oct 6;11(1):161. doi: 10.1186/s40478-023-01655-1. Acta Neuropathol Commun. 2023. PMID: 37803326 Free PMC article.
-
Development of MAPT S305 mutation human iPSC lines exhibiting elevated 4R tau expression and functional alterations in neurons and astrocytes.Cell Rep. 2024 Dec 24;43(12):115013. doi: 10.1016/j.celrep.2024.115013. Epub 2024 Nov 27. Cell Rep. 2024. PMID: 39602304
-
Elevated 4R-tau in astrocytes from asymptomatic carriers of the MAPT 10+16 intronic mutation.J Cell Mol Med. 2022 Feb;26(4):1327-1331. doi: 10.1111/jcmm.17136. Epub 2021 Dec 24. J Cell Mol Med. 2022. PMID: 34951131 Free PMC article.
-
Astroglial contribution to tau-dependent neurodegeneration.Biochem J. 2019 Nov 29;476(22):3493-3504. doi: 10.1042/BCJ20190506. Biochem J. 2019. PMID: 31774919 Review.
-
Protein astrogliopathies in human neurodegenerative diseases and aging.Brain Pathol. 2017 Sep;27(5):675-690. doi: 10.1111/bpa.12536. Brain Pathol. 2017. PMID: 28805003 Free PMC article. Review.
Cited by
-
Antisense oligonucleotides provide optimism to the therapeutic landscape for tauopathies.Neural Regen Res. 2025 Mar 1;20(3):803-804. doi: 10.4103/NRR.NRR-D-23-02057. Epub 2024 May 13. Neural Regen Res. 2025. PMID: 38886947 Free PMC article. No abstract available.
-
Tau is required for glial lipid droplet formation and resistance to neuronal oxidative stress.Nat Neurosci. 2024 Oct;27(10):1918-1933. doi: 10.1038/s41593-024-01740-1. Epub 2024 Aug 26. Nat Neurosci. 2024. PMID: 39187706 Free PMC article.
-
Elevated 4R tau contributes to endolysosomal dysfunction and neurodegeneration in VCP-related frontotemporal dementia.Brain. 2024 Mar 1;147(3):970-979. doi: 10.1093/brain/awad370. Brain. 2024. PMID: 37882537 Free PMC article.
-
Determinants of astrocytic pathology in stem cell models of primary tauopathies.Acta Neuropathol Commun. 2023 Oct 6;11(1):161. doi: 10.1186/s40478-023-01655-1. Acta Neuropathol Commun. 2023. PMID: 37803326 Free PMC article.
-
Astrocyte tau deposition in progressive supranuclear palsy is associated with dysregulation of MAPT transcription.Acta Neuropathol Commun. 2024 Aug 14;12(1):132. doi: 10.1186/s40478-024-01844-6. Acta Neuropathol Commun. 2024. PMID: 39138580 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

