Dried blood spot specimens for SARS-CoV-2 antibody testing: A multi-site, multi-assay comparison
- PMID: 34874948
- PMCID: PMC8651133
- DOI: 10.1371/journal.pone.0261003
Dried blood spot specimens for SARS-CoV-2 antibody testing: A multi-site, multi-assay comparison
Abstract
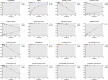
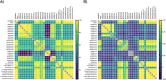
The true severity of infection due to COVID-19 is under-represented because it is based on only those who are tested. Although nucleic acid amplifications tests (NAAT) are the gold standard for COVID-19 diagnostic testing, serological assays provide better population-level SARS-CoV-2 prevalence estimates. Implementing large sero-surveys present several logistical challenges within Canada due its unique geography including rural and remote communities. Dried blood spot (DBS) sampling is a practical solution but comparative performance data on SARS-CoV-2 serological tests using DBS is currently lacking. Here we present test performance data from a well-characterized SARS-CoV-2 DBS panel sent to laboratories across Canada representing 10 commercial and 2 in-house developed tests for SARS-CoV-2 antibodies. Three commercial assays identified all positive and negative DBS correctly corresponding to a sensitivity, specificity, positive predictive value, and negative predictive value of 100% (95% CI = 72.2, 100). Two in-house assays also performed equally well. In contrast, several commercial assays could not achieve a sensitivity greater than 40% or a negative predictive value greater than 60%. Our findings represent the foundation for future validation studies on DBS specimens that will play a central role in strengthening Canada's public health policy in response to COVID-19.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Detection of SARS-CoV-2 IgG antibodies in dried blood spots.Diagn Microbiol Infect Dis. 2021 Sep;101(1):115425. doi: 10.1016/j.diagmicrobio.2021.115425. Epub 2021 May 13. Diagn Microbiol Infect Dis. 2021. PMID: 34116343 Free PMC article.
-
Simple, sensitive, specific self-sampling assay secures SARS-CoV-2 antibody signals in sero-prevalence and post-vaccine studies.Sci Rep. 2022 Feb 3;12(1):1885. doi: 10.1038/s41598-022-05640-x. Sci Rep. 2022. PMID: 35115570 Free PMC article.
-
Validation of dried blood spot sampling for detecting SARS-CoV-2 antibodies and total immunoglobulins in a large cohort of asymptomatic young adults.J Immunol Methods. 2023 Jul;518:113492. doi: 10.1016/j.jim.2023.113492. Epub 2023 May 16. J Immunol Methods. 2023. PMID: 37201783 Free PMC article.
-
Use of dried blood spots in the detection of coronavirus disease 2019 (COVID-19): A systematic review.Indian J Med Microbiol. 2024 Sep-Oct;51:100700. doi: 10.1016/j.ijmmb.2024.100700. Epub 2024 Aug 13. Indian J Med Microbiol. 2024. PMID: 39127256
-
COVID-19 new diagnostics development: novel detection methods for SARS-CoV-2 infection and considerations for their translation to routine use.Curr Opin Pulm Med. 2021 May 1;27(3):155-162. doi: 10.1097/MCP.0000000000000768. Curr Opin Pulm Med. 2021. PMID: 33654014 Review.
Cited by
-
A bead-based multiplex assay covering all coronaviruses pathogenic for humans for sensitive and specific surveillance of SARS-CoV-2 humoral immunity.Sci Rep. 2023 Dec 9;13(1):21846. doi: 10.1038/s41598-023-48581-9. Sci Rep. 2023. PMID: 38071261 Free PMC article.
-
Kinetics of dried blood spot-measured anti-SARS-CoV2 Spike IgG in mRNA-vaccinated healthcare workers.Front Microbiol. 2023 Mar 1;14:1130677. doi: 10.3389/fmicb.2023.1130677. eCollection 2023. Front Microbiol. 2023. PMID: 36937271 Free PMC article.
-
Seropositivity and risk factors for SARS-CoV-2 infection in a South Asian community in Ontario: a cross-sectional analysis of a prospective cohort study.CMAJ Open. 2022 Jul 5;10(3):E599-E609. doi: 10.9778/cmajo.20220031. Print 2022 Jul-Sep. CMAJ Open. 2022. PMID: 35790229 Free PMC article.
-
Cohort profile: investigating SARS-CoV-2 infection and the health and psychosocial impact of the COVID-19 pandemic in the Canadian CHILD Cohort.Epidemiol Health. 2023;45:e2023091. doi: 10.4178/epih.e2023091. Epub 2023 Oct 13. Epidemiol Health. 2023. PMID: 37857338 Free PMC article.
-
Does school reopening affect SARS-CoV-2 seroprevalence among school-age children in Milan?PLoS One. 2021 Sep 2;16(9):e0257046. doi: 10.1371/journal.pone.0257046. eCollection 2021. PLoS One. 2021. PMID: 34473776 Free PMC article.
References
-
- Centre for Systems and Science Engineering at John Hopkins University. COVID-19 dashboard [cited 2021 January 19]. Available from: https://coronavirus.jhu.edu/map.html.
-
- Mallett S, Allen AJ, Graziadio S, Taylor SA, Sakai NS, Green K, et al.. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Medicine. 2020;18(1):346. doi: 10.1186/s12916-020-01810-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

