Dried blood spot specimens for SARS-CoV-2 antibody testing: A multi-site, multi-assay comparison
- PMID: 34874948
- PMCID: PMC8651133
- DOI: 10.1371/journal.pone.0261003
Dried blood spot specimens for SARS-CoV-2 antibody testing: A multi-site, multi-assay comparison
Abstract
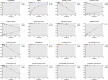
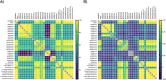
The true severity of infection due to COVID-19 is under-represented because it is based on only those who are tested. Although nucleic acid amplifications tests (NAAT) are the gold standard for COVID-19 diagnostic testing, serological assays provide better population-level SARS-CoV-2 prevalence estimates. Implementing large sero-surveys present several logistical challenges within Canada due its unique geography including rural and remote communities. Dried blood spot (DBS) sampling is a practical solution but comparative performance data on SARS-CoV-2 serological tests using DBS is currently lacking. Here we present test performance data from a well-characterized SARS-CoV-2 DBS panel sent to laboratories across Canada representing 10 commercial and 2 in-house developed tests for SARS-CoV-2 antibodies. Three commercial assays identified all positive and negative DBS correctly corresponding to a sensitivity, specificity, positive predictive value, and negative predictive value of 100% (95% CI = 72.2, 100). Two in-house assays also performed equally well. In contrast, several commercial assays could not achieve a sensitivity greater than 40% or a negative predictive value greater than 60%. Our findings represent the foundation for future validation studies on DBS specimens that will play a central role in strengthening Canada's public health policy in response to COVID-19.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Centre for Systems and Science Engineering at John Hopkins University. COVID-19 dashboard [cited 2021 January 19]. Available from: https://coronavirus.jhu.edu/map.html.
-
- Mallett S, Allen AJ, Graziadio S, Taylor SA, Sakai NS, Green K, et al. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Medicine. 2020;18(1):346. doi: 10.1186/s12916-020-01810-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

