Novel microarchitecture of human endometrial glands: implications in endometrial regeneration and pathologies
- PMID: 34875046
- PMCID: PMC8888994
- DOI: 10.1093/humupd/dmab039
Novel microarchitecture of human endometrial glands: implications in endometrial regeneration and pathologies
Abstract
Background: Human endometrium remains a poorly understood tissue of the female reproductive tract. The superficial endometrial functionalis, the site of embryo implantation, is repeatedly shed with menstruation, and the stem cell-rich deeper basalis is postulated to be responsible for the regeneration of the functionalis. Two recent manuscripts have demonstrated the 3D architecture of endometrial glands. These manuscripts have challenged and replaced the prevailing concept that these glands end in blind pouches in the basalis layer that contain stem cells in crypts, as in the intestinal mucosa, providing a new paradigm for endometrial glandular anatomy. This necessitates re-evaluation of the available evidence on human endometrial regeneration in both health and disease in the context of this previously unknown endometrial glandular arrangement.
Objective and rationale: The aim of this review is to determine if the recently discovered glandular arrangement provides plausible explanations for previously unanswered questions related to human endometrial biology. Specifically, it will focus on re-appraising the theories related to endometrial regeneration, location of stem/progenitor cells and endometrial pathologies in the context of this recently unravelled endometrial glandular organization.
Search methods: An extensive literature search was conducted from inception to April 2021 using multiple databases, including PubMed/Web of Science/EMBASE/Scopus, to select studies using keywords applied to endometrial glandular anatomy and regeneration, and the references included in selected publications were also screened. All relevant publications were included.
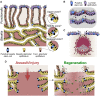
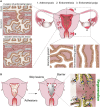
Outcomes: The human endometrial glands have a unique and complex architecture; branched basalis glands proceed in a horizontal course adjacent to the myometrium, as opposed to the non-branching, vertically coiled functionalis glands, which run parallel to each other as is observed in intestinal crypts. This complex network of mycelium-like, interconnected basalis glands is demonstrated to contain endometrial epithelial stem cells giving rise to single, non-branching functionalis glands. Several previous studies that have tried to confirm the existence of epithelial stem cells have used methodologies that prevent sampling of the stem cell-rich basalis. More recent findings have provided insight into the efficient regeneration of the human endometrium, which is preferentially evolved in humans and menstruating upper-order primates.
Wider implications: The unique physiological organization of the human endometrial glandular element, its relevance to stem cell activity and scarless endometrial regeneration will inform reproductive biologists and clinicians to direct their future research to determine disease-specific alterations in glandular anatomy in a variety of endometrial pathological conditions.
Keywords: 3D microarchitecture; adenomyosis; endometrial polyps; endometrial stem progenitor cells; endometriosis; endometrium; fertility; placenta accreta spectrum; regeneration.
© The Author(s) 2021. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures


References
-
- Al-Jefout M, Black K, Schulke L, Berbic M, Luscombe G, Tokushige N, Manconi F, Markham R, Fraser IS.. Novel finding of high density of activated mast cells in endometrial polyps. Fertil Steril 2009;92:1104–1106. - PubMed
-
- Amin TN, Saridogan E, Jurkovic D.. Ultrasound and intrauterine adhesions: a novel structured approach to diagnosis and management. Ultrasound Obstet Gynecol 2015;46:131–139. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

