Long-acting capsid inhibitor protects macaques from repeat SHIV challenges
- PMID: 34875675
- PMCID: PMC8753592
- DOI: 10.1038/s41586-021-04279-4
Long-acting capsid inhibitor protects macaques from repeat SHIV challenges
Abstract
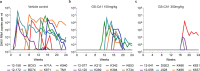
Because no currently available vaccine can prevent HIV infection, pre-exposure prophylaxis (PrEP) with antiretrovirals (ARVs) is an important tool for combating the HIV pandemic1,2. Long-acting ARVs promise to build on the success of current PrEP strategies, which must be taken daily, by reducing the frequency of administration3. GS-CA1 is a small-molecule HIV capsid inhibitor with picomolar antiviral potency against a broad array of HIV strains, including variants resistant to existing ARVs, and has shown long-acting therapeutic potential in a mouse model of HIV infection4. Here we show that a single subcutaneous administration of GS-CA1 provides long-term protection against repeated rectal simian-human immunodeficiency virus (SHIV) challenges in rhesus macaques. Whereas all control animals became infected after 15 weekly challenges, a single 300 mg kg-1 dose of GS-CA1 provided per-exposure infection risk reduction of 97% for 24 weeks. Pharmacokinetic analysis showed a correlation between GS-CA1 plasma concentration and protection from SHIV challenges. GS-CA1 levels greater than twice the rhesus plasma protein-adjusted 95% effective concentration conferred 100% protection in this model. These proof-of-concept data support the development of capsid inhibitors as a novel long-acting PrEP strategy in humans.
© 2021. The Author(s).
Conflict of interest statement
E.B., D.H., B.L., K.W., J.M., W.R., F.C., J.Z., D.K., C.C., W.B., T.C., R.G. and S.R.Y. are employees of Gilead Sciences and received salary and stock ownership as compensation for their employment. The authors otherwise declare no potential conflicts of interest.
Figures






Comment in
-
Refining HIV pre-exposure prophylactic agents.Nat Rev Drug Discov. 2022 Feb;21(2):96. doi: 10.1038/d41573-022-00014-4. Nat Rev Drug Discov. 2022. PMID: 35027695 No abstract available.
-
In PrEP: Long-acting antivirals for HIV prevention.Cell Host Microbe. 2022 Feb 9;30(2):148-150. doi: 10.1016/j.chom.2022.01.012. Cell Host Microbe. 2022. PMID: 35143766
References
-
- World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach, 2nd ed. (World Health Organization Press, 2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

