Intricate crosstalk between MYB and noncoding RNAs in cancer
- PMID: 34876130
- PMCID: PMC8650324
- DOI: 10.1186/s12935-021-02362-4
Intricate crosstalk between MYB and noncoding RNAs in cancer
Abstract
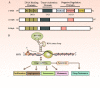
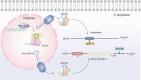
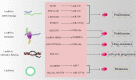
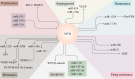
MYB is often overexpressed in malignant tumors and plays a carcinogenic role in the initiation and development of cancer. Deletion of the MYB regulatory C-terminal domain may be a driving mutation leading to tumorigenesis, therefore, different tumor mechanisms produce similar MYB proteins. As MYB is a transcription factor, priority has been given to identifying the genes that it regulates. All previous attention has been focused on protein-coding genes. However, an increasing number of studies have suggested that MYB can affect the complexity of cancer progression by regulating tumor-associated noncoding RNAs (ncRNAs), such as microRNAs, long-non-coding RNAs and circular RNAs. ncRNAs can regulate the expression of numerous downstream genes at the transcription, RNA processing and translation levels, thereby having various biological functions. Additionally, ncRNAs play important roles in regulating MYB expression. This review focuses on the intricate crosstalk between oncogenic MYB and ncRNAs, which play a pivotal role in tumorigenesis, including proliferation, apoptosis, angiogenesis, metastasis, senescence and drug resistance. In addition, we discuss therapeutic strategies for crosstalk between MYB and ncRNAs to prevent the occurrence and development of cancer.
Keywords: LncRNAs; MYB; MiRNAs; Noncoding RNAs; Tumorigenesis.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Lipsick JS, Manak J, Mitiku N, Chen CK, Fogarty P, Guthrie E. Functional evolution of the Myb oncogene family. Blood Cells Mol Dis. 2001;27(2):456–458. - PubMed
-
- Brill LB, 2nd, Kanner WA, Fehr A, Andren Y, Moskaluk CA, Loning T, Stenman G, Frierson HF., Jr Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24(9):1169–1176. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

