CircDOCK1 promotes the tumorigenesis and cisplatin resistance of osteogenic sarcoma via the miR-339-3p/IGF1R axis
- PMID: 34876132
- PMCID: PMC8650521
- DOI: 10.1186/s12943-021-01453-0
CircDOCK1 promotes the tumorigenesis and cisplatin resistance of osteogenic sarcoma via the miR-339-3p/IGF1R axis
Abstract
Background: Circular RNAs (circRNAs), a class of noncoding RNAs (ncRNAs), may modulate gene expression by binding to miRNAs. Additionally, recent studies show that circRNAs participate in some pathological processes. However, there is a large gap in the knowledge about circDOCK1 expression and its biological functions in osteogenic sarcoma (OS).
Methods: Differentially expressed circRNAs in OS cell lines and tissues were identified by circRNA microarray analysis and quantitative real-time PCR (qRT-PCR). To explore the actions of circDOCK1 in vivo and in vitro, circDOCK1 was knocked down or overexpressed. To assess the binding and regulatory associations among miR-339-3p, circDOCK1 and IGF1R, we performed rescue experiments, RNA immunoprecipitation (RIP), RNA pulldown assays and dual-luciferase assays. Moreover, we performed apoptosis assays to reveal the regulatory effects of the circDOCK1/miR-339-3p/IGF1R axis on cisplatin sensitivity.
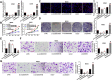
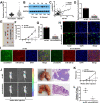
Results: CircDOCK1 expression remained stable in the cytoplasm and was higher in OS tissues and cells than in the corresponding controls. Overexpression of circDOCK1 increased oncogenicity in vivo and malignant transformation in vitro. In the U2OS and MG63 cell lines, circDOCK1 modulated tumor progression by regulating IGF1R through sponging of miR-339-3p. Additionally, in the U2OS/DDP and MG63/DDP cell lines, cisplatin sensitivity was regulated by circDOCK1 via the miR-339-3p/IGF1R axis.
Conclusions: CircDOCK1 can promote progression and regulate cisplatin sensitivity in OS via the miR-339-3p/IGF1R axis. Thus, the circDOCK1/miR-339-3p/IGF1R axis may be a key mechanism and therapeutic target in OS.
Keywords: Cisplatin resistance; IGF1R; OS; circDOCK1; miR-339-3p.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest regarding this manuscript.
Figures





Similar articles
-
circCUL2 regulates gastric cancer malignant transformation and cisplatin resistance by modulating autophagy activation via miR-142-3p/ROCK2.Mol Cancer. 2020 Nov 5;19(1):156. doi: 10.1186/s12943-020-01270-x. Mol Cancer. 2020. PMID: 33153478 Free PMC article.
-
Long non-coding RNA ROR regulated ABCB1 to induce cisplatin resistance in osteosarcoma by sponging miR-153-3p.Eur Rev Med Pharmacol Sci. 2019 Sep;23(17):7256-7265. doi: 10.26355/eurrev_201909_18828. Eur Rev Med Pharmacol Sci. 2019. PMID: 31539112
-
circ_PPAPDC1A promotes Osimertinib resistance by sponging the miR-30a-3p/ IGF1R pathway in non-small cell lung cancer (NSCLC).Mol Cancer. 2024 May 7;23(1):91. doi: 10.1186/s12943-024-01998-w. Mol Cancer. 2024. PMID: 38715012 Free PMC article.
-
Research progress on the role of lncRNA, circular RNA, and microRNA networks in regulating ferroptosis in osteosarcoma.Biomed Pharmacother. 2024 Jul;176:116924. doi: 10.1016/j.biopha.2024.116924. Epub 2024 Jun 14. Biomed Pharmacother. 2024. PMID: 38876052 Review.
-
Recent advances in the contribution of circRNAs to cisplatin chemotherapy resistance in cancers.Neoplasma. 2021 Nov;68(6):1119-1131. doi: 10.4149/neo_2021_210624N846. Epub 2021 Sep 16. Neoplasma. 2021. PMID: 34533032 Review.
Cited by
-
Circ_0002669 promotes osteosarcoma tumorigenesis through directly binding to MYCBP and sponging miR-889-3p.Biol Direct. 2024 Apr 3;19(1):25. doi: 10.1186/s13062-024-00466-1. Biol Direct. 2024. PMID: 38570856 Free PMC article.
-
Single-cell aggrephagy-related patterns facilitate tumor microenvironment intercellular communication, influencing osteosarcoma progression and prognosis.Apoptosis. 2024 Apr;29(3-4):521-535. doi: 10.1007/s10495-023-01922-5. Epub 2023 Dec 8. Apoptosis. 2024. PMID: 38066392
-
Cuproptosis signature and PLCD3 predicts immune infiltration and drug responses in osteosarcoma.Front Oncol. 2023 Mar 16;13:1156455. doi: 10.3389/fonc.2023.1156455. eCollection 2023. Front Oncol. 2023. PMID: 37007130 Free PMC article.
-
LncRNA mediated metabolic reprogramming: the chief culprits of solid tumor malignant progression: an update review.Nutr Metab (Lond). 2024 Nov 8;21(1):89. doi: 10.1186/s12986-024-00866-0. Nutr Metab (Lond). 2024. PMID: 39516895 Free PMC article. Review.
-
Permeable Hydrogel Encapsulated Osteosarcoma-on-a-Chip for High-Throughput Multi-Drugs Screening.Smart Med. 2025 Jul 12;4(3):e70013. doi: 10.1002/smmd.70013. eCollection 2025 Sep. Smart Med. 2025. PMID: 40662054 Free PMC article.
References
-
- Zhang H, Wang J, Ren T, Huang Y, Liang X, Yu Y, Wang W, Niu J, Guo W. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020;490:54–65. - PubMed
-
- Versleijen-Jonkers YM, Vlenterie M, van de Luijtgaarden AC, van der Graaf WT. Anti-angiogenic therapy, a new player in the field of sarcoma treatment. Crit Rev Oncol Hematol. 2014;91:172–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

