Renal function in Japanese HIV-1-positive patients who switch to tenofovir alafenamide fumarate after long-term tenofovir disoproxil fumarate: a single-center observational study
- PMID: 34876151
- PMCID: PMC8650504
- DOI: 10.1186/s12981-021-00420-5
Renal function in Japanese HIV-1-positive patients who switch to tenofovir alafenamide fumarate after long-term tenofovir disoproxil fumarate: a single-center observational study
Abstract
Background: Tenofovir disoproxil fumarate (TDF) has a strong antiviral effect, but TDF is known to cause renal dysfunction. Therefore, we are investigating preventing renal dysfunction by replacing TDF with tenofovir alafenamide fumarate (TAF), which is known to be relatively safe to the kidneys. However, the changes in renal function under long-term use of TAF are not known. In this study, we evaluated renal function in Japanese HIV-1-positive patients switching to TAF after long-term treatment with TDF.
Methods: A single-center observational study was conducted in Japanese HIV-1-positive patients. TDF was switched to TAF after at least 48 weeks of the treatment so we could evaluate the long-term use of TDF. The primary endpoint was the estimated glomerular filtration rate (eGFR) at 144 weeks of TAF administration. In addition, we predicted the factors that would lead to changes in eGFR after long-term use of TAF.
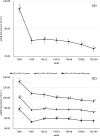
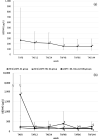
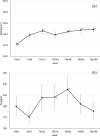
Results: Of the 125 HIV-1-positive patients who were prescribed TAF at our hospital during the study period, 70 fulfilled the study criteria. The eGFR at the time of switching from TDF to TAF was 81.4 ± 21.1 mL/min/1.73 m2. eGFR improved significantly after 12 weeks of taking TAF but significantly decreased at 96 and 144 weeks. The factors significantly correlated with the decrease in eGFR at 144 weeks on TAF were eGFR and weight at the start of TAF.
Conclusions: In this study, it was confirmed that switching to TAF was effective for Japanese HIV-1-positive patients who had been taking TDF for a long period of time and had a reduced eGFR. It was also found that the transition status depended on the eGFR and weight at the time of switch. Since HIV-1-positive patients in Japan are expected to continue taking TAF for a long time, renal function and body weight should be carefully monitored.
Keywords: HIV; Renal function; Tenofovir alafenamide fumarate; Tenofovir disoproxil fumarate; eGFR.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Renal function and lipid metabolism in Japanese HIV-1-positive individuals 288 weeks after switching from tenofovir disoproxil fumarate to tenofovir alafenamide fumarate: a single-center, retrospective cohort study.J Pharm Health Care Sci. 2024 Feb 28;10(1):13. doi: 10.1186/s40780-024-00336-y. J Pharm Health Care Sci. 2024. PMID: 38419093 Free PMC article.
-
Effect of switching from tenofovir disoproxil fumarate to tenofovir alafenamide on estimated glomerular filtration rate slope in patients with HIV: A retrospective observational study.J Infect Chemother. 2022 Mar;28(3):396-400. doi: 10.1016/j.jiac.2021.11.016. Epub 2021 Dec 9. J Infect Chemother. 2022. PMID: 34896027
-
Changes in alanine aminotransferase levels after switching from tenofovir disoproxil fumarate (TDF) to tenofovir alafenamide (TAF) in HIV-positive people without viral hepatitis in the Swiss HIV Cohort Study.HIV Med. 2021 Aug;22(7):623-628. doi: 10.1111/hiv.13106. Epub 2021 Apr 20. HIV Med. 2021. PMID: 33880839
-
Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: A meta-analysis of randomized controlled trials.Int J Infect Dis. 2020 Apr;93:108-117. doi: 10.1016/j.ijid.2020.01.035. Epub 2020 Jan 25. Int J Infect Dis. 2020. PMID: 31988012 Review.
-
Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF).Biochem Pharmacol. 2016 Nov 1;119:1-7. doi: 10.1016/j.bcp.2016.04.015. Epub 2016 Apr 29. Biochem Pharmacol. 2016. PMID: 27133890 Review.
Cited by
-
Risk of dyslipidaemia in people living with HIV who are taking tenofovir alafenamide: a systematic review and meta-analysis.J Int AIDS Soc. 2024 Sep;27(9):e26358. doi: 10.1002/jia2.26358. J Int AIDS Soc. 2024. PMID: 39301685 Free PMC article.
-
Treatment persistence of bictegravir/emtricitabine/tenofovir alafenamide and efavirenz + lamivudine + tenofovir disoproxil among HIV-1 patients newly starting treatment in Hunan Province in China.BMC Infect Dis. 2023 Jun 12;23(1):396. doi: 10.1186/s12879-023-08359-w. BMC Infect Dis. 2023. PMID: 37308847 Free PMC article.
-
Effect of Entecavir, Tenofovir Disoproxil Fumarate, and Tenofovir Alafenamideantiviral Therapy on Renal Function in Chronic Hepatitis B Patients: A Real-World Retrospective Study.Int J Gen Med. 2025 Feb 27;18:1143-1153. doi: 10.2147/IJGM.S497550. eCollection 2025. Int J Gen Med. 2025. PMID: 40034830 Free PMC article.
-
Renal function and lipid metabolism in Japanese HIV-1-positive individuals 288 weeks after switching from tenofovir disoproxil fumarate to tenofovir alafenamide fumarate: a single-center, retrospective cohort study.J Pharm Health Care Sci. 2024 Feb 28;10(1):13. doi: 10.1186/s40780-024-00336-y. J Pharm Health Care Sci. 2024. PMID: 38419093 Free PMC article.
References
-
- World Health Organization. Interim guidelines on HIV/AIDS. 2018. https://apps.who.int/iris/bitstream/handle/10665/277395/WHO-CDS-HIV-18.5.... Revised on October, 2021.
-
- U.S. Department of Health & Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2021. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Ad.... Revised on October, 2021.
-
- European AIDS Clinical Society. Guidelines version 11.0. 2021. https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf. Revised on October, 2021.
-
- Casado JL, del Rey JM, Bañón S, et al. Changes in kidney function and in the rate of tubular dysfunction after tenofovir withdrawal or continuation in HIV-infected patients. JAIDS. 2016;72:416–422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

